News and Events
Proteins Released from the Nuclei of Dying Cancer Cells Promote Tumor Growth
Material released from dying cancer cells, known as tumor cell nuclear expulsion products (TuNEPs), contains specific proteins that promote the growth of neighboring cancer cells. Targeting these proteins could lead to new treatments that hinder cancer spread and improve patient outcomes.
Read MoreClinical trial evaluates a combination therapy for metastatic clear cell renal carcinoma
Kidney cancer is the 6th most common cancer diagnosed in the United States with greater than 73,000 new cases seen each year. CCR investigators are interested in studying a combination immunotherapy for its most common subtype, clear cell renal carcinoma, using Avelumab and interleukin (IL)-15.
Read MoreIn Memoriam: Larry K. Keefer, Ph.D.
The Center for Cancer Research mourns the recent death of past colleague Larry K. Keefer. He made major contributions to the understanding of the chemistry of the carcinogenic nitrosamines.
Read MoreClinical trial evaluates immunotherapy for head and neck cancer caused by HPV
A clinical trial, led by Christian Hinrichs, studies neoadjuvant immunotherapy for HPV-related head and neck cancer. Neoadjuvant means it is given before main treatments such surgery. The goal of the study is to see if T cells given before the main treatment can reduce the risk of the disease coming back and to convert borderline or unresectable tumors to resectable.
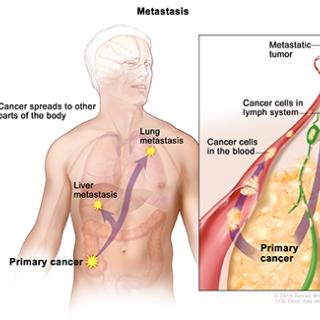
Read MoreTrial opens to evaluate a potential anti-metastasis compound
The Center for Cancer Research has opened a phase I trial to evaluate the safety and clinical activity of metarrestin, a compound that suppressed metastasis and extended survival in several preclinical cancer models.
Read MoreClinical trial will evaluate chemotherapy-sparing approach to treating Kaposi sarcoma
CCR physician-scientists have launched a clinical trial to evaluate whether the immune-stimulating molecule NHS-IL12, alone or in combination with an experimental immunotherapy called M7824, reduces tumors in patients with Kaposi sarcoma.
Read MoreUndergraduate research at NCI sparks curiosity and new connections
Brandon Ogbunu, a Professor of Ecology and Evolutionary Biology at Yale, recalls the time he spent studying gene regulation in Susan Gottesman’s lab. He says the experience sparked interests that have helped shape his career studying evolution and disease.
Read MoreInnovative imaging tools reveal how neutrophils fight inflammation in mice
CCR researchers used state-of-the-art imaging techniques to observe, in real time, the path neutrophils take as they move toward a site of inflammation in a mouse model. The images showed the neutrophils leaving the blood vessels and engaging with bacteria that had been introduced into the mouse’s footpad. These observations led to the team’s discovery that the leukocyte LTB4 directs the recruitment, engagement, and penetration of neutrophils into inflamed tissues.
Read MoreFDA grants rare pediatric disease designation for experimental immunotherapy for acute lymphoblastic leukemia
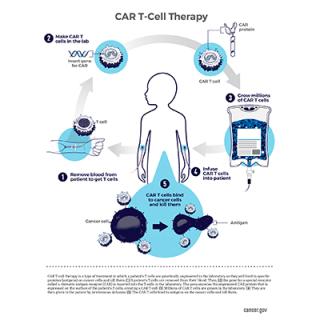
A cell-based immunotherapy that is currently being evaluated at the NIH Clinical Center for the treatment of B-cell acute lymphoblastic leukemia (ALL) has been designated by the U.S. Food and Drug Administration as a drug for a rare pediatric disease. Designation could encourage development of this novel therapy for children with relapsed or refractory ALL.
Read MoreIn mice, new therapeutic strategy enhances the effects of immunotherapy in high ASS1-expressing cancers
Many prevalent cancers (e.g., lung, breast and colon) exhibit abnormally high expression of the enzyme ASS1. This results in greater production of genetic material incorporating purines, consequently making these tumors more resistant to immunotherapy. The researchers showed that drugs blocking purine production in these tumors enhance their response to immunotherapy.
Read MoreExperimental compound blocks SARS-CoV-2’s ability to infect and kill cells in the lab
A potential therapy for COVID-19 binds to a viral enzyme that SARS-CoV-2 requires for replication. With further development and testing, it might be an effective treatment for people with the disease.
Read More