News and Events
New Strategy Shows Promise Against Deadly Breast Cancer in the Brain
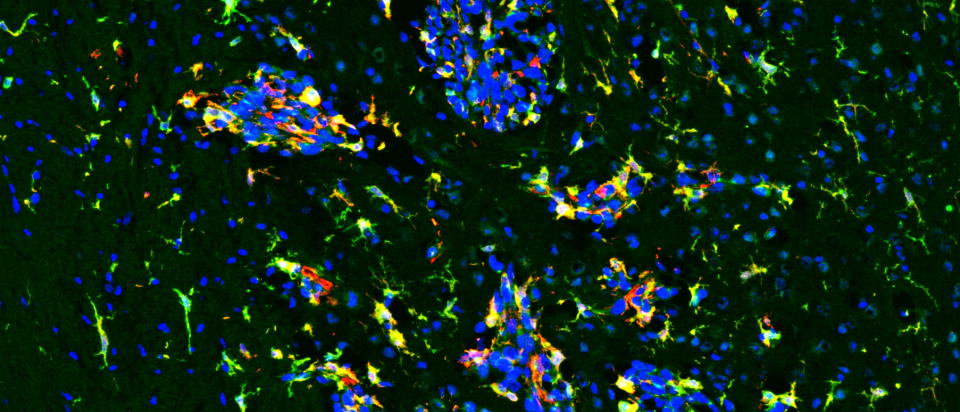
A new NIH study points to a promising strategy for treating aggressive breast cancer that spreads to the brain, a complication with few effective options. Learn how blocking a key brain cell survival pathway could open the door to future therapies.
Read MoreClinical trial studies therapy for relapsed/refractory T-cell malignancies
Milos Miljkovic, M.D., M.Sc., Assistant Research Physician in the Lymphoid Malignancies Branch, is leading a study of a 4-drug combination treatment strategy using romidepsin, oral 5-azacitidine, dexamethasone and lenalidomide for T-cell malignancies (TCMs). Researchers are seeking to determine the safety, side effects, and best dose of this 4-drug combination for people with relapsed/refractory TCM.
Read MoreStudy evaluates, treats, and follows patients with gastrointestinal stromal tumors
More than half of all gastrointestinal stromal tumors (GISTs) start in the stomach, but they can start anywhere in the GI tract. Andrew M. Blakely, M.D., Assistant Research Physician in the Surgical Oncology Program, is leading a study of GIST that could benefit current and future patients.
Read MoreNew clinical trial studies immunotherapy combination for metastatic breast cancer
Fatima Karzai, M.D., Associate Research Physician in the Genitourinary Malignancies Branch, is leading a study in adults with breast cancer that has spread to other places in the body. Researchers want to see if a combination of four drugs, which includes immunotherapy, can shrink the tumors of metastatic breast cancer.
Read MoreClinical trial studies therapy for adults with BRAF-mutant hairy cell leukemia
Adults with BRAF-mutant hairy cell leukemia, that did not respond to or came back after treatment, may be eligible to participate in a clinical trial at the NIH Clinical Center. Researchers want to see if combining encorafenib and binimetinib has an improved response rate than other drugs previously used to treat HCL.
Read MoreNew connection between gut microbes and liver cancer uncovered in mice
Researchers have uncovered a mechanism by which gut microbes can influence the immune response against nearby liver tumors in mice. The new findings could potentially explain why some people with liver or gut diseases, such as primary sclerosing cholangitis and colitis, tend to be more susceptible to cholangiocarcinoma.
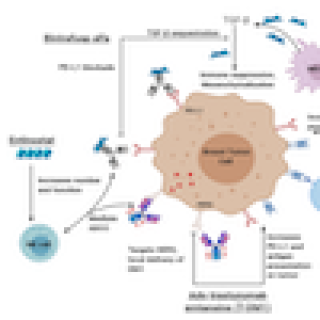
Read MoreImmunotherapy combination tested for advanced/metastatic solid tumors
Adults with locally advanced or metastatic solid tumors that cannot be treated with surgery may be eligible to participate in a clinical trial at the NIH Clinical Center. James L. Gulley, M.D., Ph.D., Chief of the Genitourinary Malignancies Branch, is leading a study exploring several different combinations of immunotherapies to see if they improve responses in people with cancer.
Read MoreAnalysis of T cells from melanoma patients uncovers characteristics leading to cancer regression
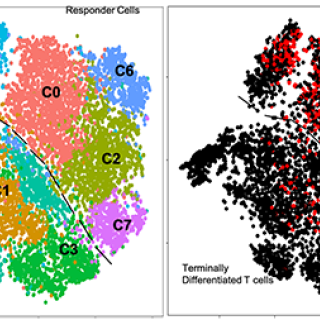
CCR researchers used state-ot-the-art analytic techniques to determine characteristics of cells used in effective adoptive T cell therapy (ACT) in patients with advanced melanoma. This new information opens the door to manufacturing these cells in the laboratory and using them in immunotherapy for common cancers, including liver, breast, prostate, and colon cancer.
Read MoreClinical trial evaluates therapy for adults with relapsed/refractory HCL
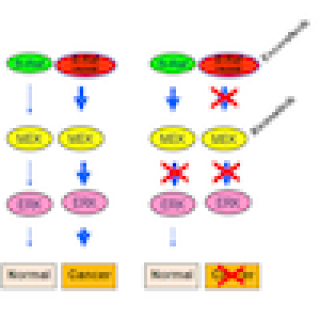
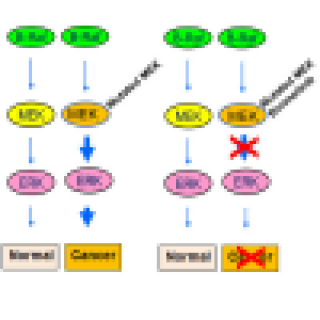
Robert J. Kreitman, M.D., Senior Investigator in the Laboratory of Molecular Biology, is leading a study of a treatment for hairy cell leukemia (HCL). The goal of this study is to determine the overall rate of response to binimetinib, an MEK inhibitor, in patients with HCL who do not have a BRAF gene mutation.
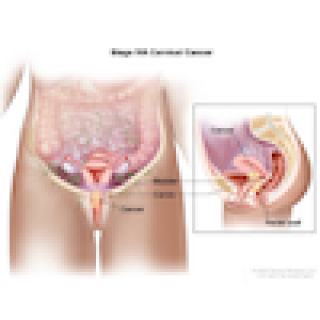
Read MoreTreatment for women with advanced, untreated cervical cancer tested in new clinical trial
Women 18 and older with previously untreated advanced cervical cancer may be eligible to participate in a clinical trial at the NIH Clinical Center. Christian S. Hinrichs, M.D., Senior Investigator in the Genitourinary Malignancies Branch, is leading a study of a treatment called induction immunotherapy that uses the patient’s own T cells (immune cells) to attack their cancer cells.
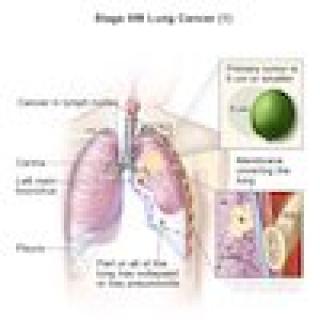
Read MoreClinical trial studies drug combination for EGFR-mutated adenocarcinomas
Adults with epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer that has transformed to small cell lung cancer or a neuroendocrine tumor may be eligible to participate in a clinical trial at the NIH Clinical Center. Anish Thomas, M.B.B.S., M.D., NIH Lasker Clinical Research Scholar in the Developmental Therapeutics Branch, is leading a trial testing a drug combination to treat these kinds of tumors.
Read More