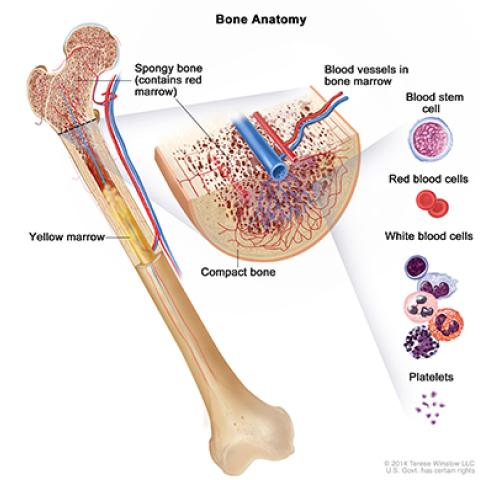
Bone Anatomy
Image Source: NCI Visuals Online
People with newly diagnosed moderate or severe chronic graft-versus-host-disease (cGvHD) may be eligible to participate in a clinical trial at the NIH Clinical Center.
Steven Pavletic, M.D., M.S., Senior Clinician in the Immune Deficiency Cellular Therapy Program, is leading a trial of the lymphoma therapy drug ibrutinib for patients with newly diagnosed cGvHD. cGvHD can occur after a person has had a stem cell or bone marrow transplant. In some cases, the donated bone marrow/stem cells view the host's body as foreign and start to attack it. cGvHD can occur at any time after a transplant, but it's more common after the marrow/stem cells have created a new immune system in the host's body. cGvHD may be mild or severe and may last for a long time. Ibrutinib works by blocking the action of the abnormal protein that signals lymphoma cancer cells to multiply. This helps to stop the spread of cancer cells. Researchers want to see if early treatment with ibrutinib can prevent the most severe and irreversible symptoms of cGvHD. The hope is to avoid administration of corticosteroids, which are now used as standard therapy for newly diagnosed cGvHD.
Clinicaltrials.gov identifier: NCI-20-C-0058
NCI Protocol ID: NCT04294641
Official Title: A Pilot Study of Front Line Ibrutinib for Newly Diagnosed Chronic Graft-Versus-Host Disease
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.