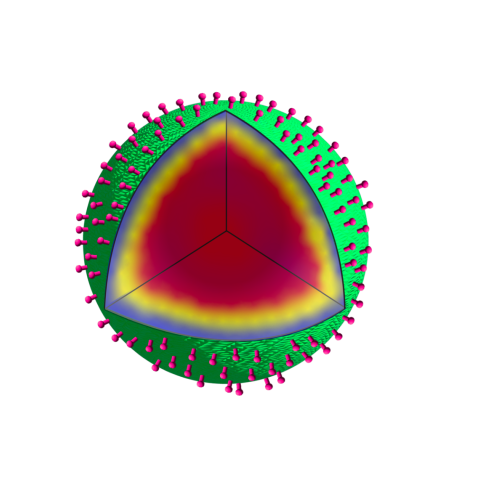
Three-dimensional illustration of a SSHEL drug delivery system. An inner porous silica-based cavity carrying cargo (dark red) is coated by a membrane (yellow) and protein layers (purple, green), which serve as a platform to attach cancer-targeting molecules (pink protrusions).
CCR researchers have developed a novel way to shuttle drugs directly into cancer cells, allowing them to treat cancer more effectively and with fewer side effects. The work, published in Cell Reports and available online now, offers a new approach to preventing some potential harmful side effects of systemic chemotherapy.
Side effects of some chemotherapy drugs can range from nausea and fatigue to mood changes and nerve problems, as well as damage to healthy cells and organs. “These side effects are a huge deterrent for people getting chemotherapy,” says Kumaran S. Ramamurthi, Ph.D., Senior Investigator and Deputy Chief of the Laboratory of Molecular Biology and lead investigator of the study. “One way to solve this problem is to make sure that chemotherapy drugs reach only the cancer and not surrounding tissue.”
Inspired by nature, Ramamurthi’s team created artificial, bacterial spore-like particles that can target cancer while carrying chemotherapeutic cargo. The product, called a SSHEL (short for Synthetic Spore Husk-Encased Lipid bilayers), consists of a porous glass bead surrounded by a membrane and a protein coat dotted with cancer-targeting molecules.
To test their concept, the team collaborated with the groups of David Fitzgerald, Ph.D., Senior Investigator in the Laboratory of Molecular Biology, and Kandice Tanner, Ph.D., Senior Investigator in the Laboratory of Cell Biology, to use a mouse model of HER2-positive ovarian cancer, which is sometimes treated with a chemotherapy drug called doxorubicin. They loaded doxorubicin into SSHELs designed to home in on HER2 proteins and then injected the SSHELs into the mice. Compared to the leading doxorubicin delivery method (doxorubicin encased in a fat-based sphere), the SSHELs shrank the tumors more effectively and with fewer side effects.
In a serendipitous twist, it was the cancer cell’s own machinery that secured its demise. The team’s initial goal was to reach the cancer and then work on drug release, but when the SSHELs contacted the cancer cell surface, they were trafficked into an acidic area of the cell. This destabilized the SSHEL particle and released the doxorubicin cargo. “That was unexpected, but it was fortuitous,” Ramamurthi says.
The team confirmed their findings in a transparent zebrafish model of ovarian cancer. Using fluorescent markers, they tracked the SSHELs at the surface of cancer cells and inside acidic compartments.
Moving forward, Ramamurthi and collaborators plan to examine the utility of SSHELs in another mouse model. “This is a classic example of translating basic research into an applied setting,” Ramamurthi says. His hope is to eventually evaluate SSHELs in a clinical trial.
Ramamurthi emphasized that SSHEL relevance reaches beyond ovarian cancer and chemotherapeutic drug delivery. “We're providing a versatile platform that could be used for a lot of different applications,” he says. “Tell us what should be targeted, and we could provide a vehicle to get the cargo there.”