The metabolism of cancer cells differs markedly from that of healthy cells. It is now becoming clear that these differences may be a driving force of cancer cells. This insight will lead to new approaches to disrupt cancers cells’ metabolic pathways.
A three-dimensional illustration of a cancer cell in the process of mitosis. Credit: iStock
We have known for nearly a century that the ways cancer cells generate energy and cellular materials can differ markedly from how these functions are handled by healthy cells. Now, we are learning that metabolic changes are not just a feature of cancer cells — they may be one of their driving forces. This insight is a gateway to more effective and less toxic cancer therapies that work by disrupting specific metabolic pathways. It will also pave the way to new diagnostic tools to help guide treatment decisions for patients with cancer.
The complex metabolic pathways of cells work together to convert food into energy and chemical compounds. Cancer cells often abandon the efficient energy-producing pathways used by healthy cells and shift to alternative strategies that generate more of the materials they need to proliferate. Although this shift provides a growth advantage to cancer cells, it also represents a vulnerability. Rapidly dividing cells can become so dependent on these alternative pathways that interfering with them might be a powerful way to thwart tumor growth.
Some genes that were tied to cancer long ago are now being found to play roles in metabolism. Known cancer-associated genes influence the way cells take up nutrients, convert food to energy and generate vital biological compounds. It is likely that there are many yet undiscovered metabolic genes and pathways that support tumor growth and survival. Identifying these culprits is important because they could be new targets for therapeutic intervention.
A first example of the importance of metabolism in cancer is the several metabolic genes discovered in CCR, that, when mutated, increase the risk of kidney cancer. Mutations in metabolic genes are now known to contribute to a wide range of cancers, including cancers of the brain, prostate, pancreas and lung. Metabolic genes are targets for novel drugs, such as Belzutifan, which was FDA-approved for patients with von Hippel-Lindau disease in 2021 based on studies performed in CCR.
CCR’s clinical metabolomics efforts take advantage of technical advances that have made it possible to detect and identify thousands of different metabolites — the chemicals generated by cellular metabolism — in tissue samples. This has created an opportunity to pinpoint specific changes to metabolic pathways inside patient tumors by comparing their metabolic profiles to those of healthy cells. Current studies aim to track how metabolism changes as cancer progresses and metastasizes or as tumors respond to anti-cancer therapy, especially during the development of drug resistance.
These metabolic landscapes will enable deeper investigations into how specific metabolic changes support tumor growth. That knowledge, in turn, will guide the development of new treatments that deprive cancer cells of biochemical resources on which they have come to depend. It might even point to existing metabolism-modifying drugs, such as those used to lower cholesterol or treat diabetes, that may become useful in treating or preventing cancer.
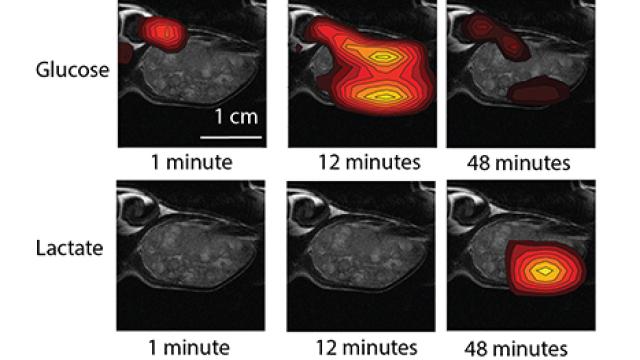
Additional opportunities arise from new clinical imaging technology that can monitor metabolism inside the body in real time. Images captured inside an MRI scanner can show where in the body a particular compound is being broken down or converted into something else. When that chemical transformation depends on a cancer-fueling metabolic pathway, such imaging could reveal the location of a tumor. As our understanding of the relationship between cancer and metabolism deepens, this type of imaging might be deployed to determine how aggressive a patient’s cancer is or to quickly assess their tumor’s response to a particular therapy, allowing patients and their doctors to make better-informed treatment decisions.