Daniel R. Larson, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 41, Room B201
- Bethesda, MD 20892
- 240-760-6653
- dan.larson@nih.gov
RESEARCH SUMMARY
The primary goal of Dan Larson's laboratory is to understand gene expression in eukaryotic cells, starting from the mechanistic behavior of individual macromolecules and proceeding to their regulation in cells and tissue. The laboratory utilizes a battery of biophysical, molecular and genomic approaches, including single-molecule microscopy, RNA visualization in fixed and living cells, computational modeling of gene regulation, and nascent RNA sequencing. Dr. Larson helped pioneer in vivo single-molecule studies of transcription and splicing. The view that has emerged from these studies is that gene regulation is a dynamic process resulting in stochastic variation within populations. Current work is focused on applying these experimental and theoretical approaches to the study of hematopoiesis in health and disease through the trans-NIH Myeloid Malignancies Program.
Areas of Expertise
Myeloid Malignancies Program
A trans-NIH multidisciplinary network of researchers and clinicians dedicated to improving early detection, diagnosis, prognosis and development of novel therapeutics for myeloid malignancies.

Daniel R. Larson, Ph.D.
Research
Gene Expression in Single Cells
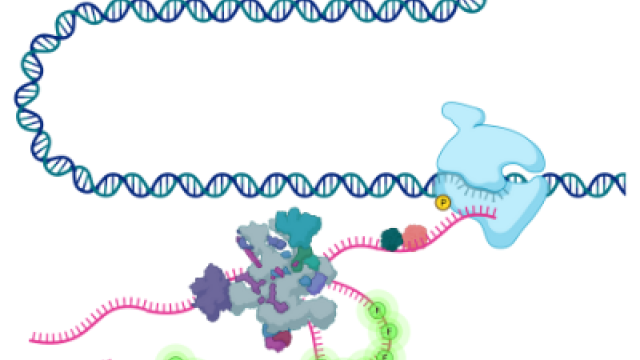
Gene expression refers to the sum of processes that enable cells to control their complement of RNA and protein. Megadalton molecular machines such as RNA polymerase, the spliceosome, and the ribosome carry out the synthesis, processing, and translation of RNA. Advances in structural biology have revealed the atomic details of these machines, and the revolution in sequencing has engendered an understanding of their respective enzymatic activities with nucleotide resolution. A central challenge in cell biology is to understand how these processes are coupled and regulated in time and space in single living cells. In recent years, through parallel advances in microscopy, fluorescent probe development, computational modeling, and gene editing it is now possible to directly observe the processes of gene expression (transcription, splicing, translation) in living cells. The Larson lab has played an essential role in this development, for example by being the first lab to visualize transcription and splicing of single human genes in real time. The view that has emerged from these studies is that gene regulation is an extremely dynamic process where the random behavior of individual molecules plays an important role in determining how a cell controls expression. Coupling live-cell imaging with RNA-sequencing based methods is an exciting approach for understanding gene expression in health and disease.
Gene Expression in Myeloid Malignancy
The differentiation of hematopoietic stem cells into committed lineages in the blood is the result of concerted regulation between transcription, splicing and translation, and a goal of the laboratory is to understand the dynamic interplay between these processes in single cells. Moreover, mutations in the gene expression machinery drive the development of clonal stem cell disorders termed ‘myeloid malignancies.’ Specifically, the Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML) are a heterogeneous group of malignant clonal hematopoietic stem cell disorders with poor prognosis and few treatment options. MDS is characterized by ineffective hematopoiesis, marrow dysplasia, peripheral blood cytopenia, and a high propensity for transformation into AML, which is an acute proliferative disease. Simply stated, MDS is a disease of differentiation, while AML is a disease of differentiation plus proliferation. Over 60% of MDS patients carry a mutation in the spliceosome, and the Larson lab has proposed a non-canonical role for the splicing machinery in disease progression. Overall, gene regulation in this tissue is exceptionally dependent on post-transcriptional mechanisms, opening new avenues of research for understanding hematopoiesis. Analysis of primary human samples and close coupling with the newly-established Myeloid Malignancies clinical program – “bench-to-bedside-to-bench” – makes this area a vibrant and impactful area of study where basic concepts in gene regulation can be immediately applied to human health.
Thus, the projects in the laboratory are loosely divided into several areas:
1) Visualizing spliceosome assembly and splice site selection
2) Deciphering the role of nuclear structure in modulating gene expression
3) Determining the causes and consequences of transcriptional heterogeneity
4) Developing in vitro reconstituted models for examining the coupling between transcription, splicing, translation
5) Computational analysis of gene expression
6) Understanding gene expression alteration in myeloid malignancy.
Resources
Publications
Intrinsic Dynamics of a Human Gene Reveal the Basis of Expression Heterogeneity.
The splicing factor U2AF1 contributes to cancer progression through a noncanonical role in translation regulation.
Single-Molecule Imaging Reveals a Switch between Spurious and Functional ncRNA Transcription
Biography

Daniel R. Larson, Ph.D.
Dr. Larson was trained in biophysics, receiving a B.S. in physics from the Ohio State University and then a Ph.D. in biophysics from Cornell University working in the laboratory of Watt W. Webb. During this time, he developed a range of optical methods for interrogating macromolecular interactions in living cells. As a joint postdoctoral fellow in the laboratories of Robert Singer and John Condeelis at Albert Einstein College of Medicine, he helped pioneer in vivo single-molecule studies of transcription. Currently, his lab focuses on single-cell analysis of gene regulation -- with an emphasis on RNA -- including transcription, splicing, and translation. There is a strong emphasis on applying these approaches to understand gene expression in hematopoiesis, particularly in the context of myeloid malignancy. In 2011, he was awarded the Presidential Early Career Award for Scientists and Engineers. An NIH Stadtman Investigator, Dr. Larson received tenure in 2019. He is currently co-chair of the trans-NIH Myeloid Malignancies Program.
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
Alumni
Covers
MYC amplifies gene expression through global changes in transcription factor dynamics
On the cover: In this issue, Patange et al. used optogenetics and fluorescence microscopy to demonstrate how the MYC oncogene amplifies transcription genome-wide by globally altering the dwell times of transcription factors. The cover depicts a microscope image of human osteosarcoma cells expressing high levels of MYC, where cell nuclei (purple) are in various states of transcribing a gene into RNA (gold). This snapshot captures an active transcription site in the center nucleus. Image by Simona Patange.
Patange S, Ball DA, Wan Y, Karpova TS, Girvan M, Levens D, Larson DR. 2022. MYC amplifies gene expression through global changes in transcription factor dynamics. Cell Reports 38.