Antiviral Immunity and Resistance Section
Alex Compton, Ph.D.
Research
Improving Human Antiviral Immunity, One Cell at a Time
The clinical outcome of viral infection, the difference between survival and death of the host, rests delicately on events occurring at the molecular level of individual cells. The "cell-intrinsic" arm of innate immunity prevents virus replication in host cells by detecting virus invasion and interfering with the viral life cycle. As such, cell-intrinsic immune factors, also known as host restriction factors, impose the earliest-acting barriers to invading pathogens. Research in this field, which relies increasingly on interdisciplinary approaches and bioinformatics, has demonstrated that the survival of single cells equates strongly with survival of the organism, and even of the population (or species) to which it belongs. Since its inception in 2017, the Compton lab focuses on mechanisms of cell-intrinsic immunity and the strategies employed by HIV and emerging viruses to evade or overcome these immune barriers.
By combining relevant experimental systems in virology with perspectives in cell biology and evolutionary biology, we aim to better understand the factors governing virus entry into cells. We employ a “cross-species” approach, in which diverse viruses are paired with host cells of diverse species in order to reveal cell-intrinsic barriers that limit virus infection. Cellular membranes, composed of proteins and lipids, are the first line of defense against infection. Residents of this critical threshold include cellular transmembrane proteins that remodel membrane vesicles or redirect their trafficking in order to inhibit the viral entry process. In addition to protecting cells from infection, cellular membrane components also impact the structure and infectivity of nascent virions produced from infected cells. Overall, cell-intrinsic immunity acting on membranes performs dual antiviral functions by 1) preventing virus infection of individual cells, and 2) limiting the spread of virus between cells.
Current projects in the lab revolve around the following themes:
1. Mechanisms of virus entry into cells and evasion of cell-intrinsic immunity
2. Enhancement of virus delivery for gene editing in human cells and in vivo
3. Signals regulating the intrinsic antiviral state: stress, metabolism, differentiation, and activation
Team
Job Vacancies
Interested in joining the Compton lab?
Postdoctoral fellows, graduate students (Ph.D., M.D./Ph.D.), postbaccalaureate fellows, and summer interns (high school, undergraduate) have been trained in the Compton lab since it opened in 2017. Interested candidates should send a CV and statement of interest to alex.compton@nih.gov.
What does the lab have to offer?
- Stable funding and multi-year appointments
- Resource-rich training environment
- Fun, culturally diverse, and interdisciplinary lab mates and department colleagues
- Exciting research spanning from basic to translational, from in vitro to in vivo
- Infinite opportunities for collaboration on a one-of-a-kind campus: the NIH Intramural Research Program is the world’s largest biomedical research institution, with 1,200 principal investigators and over 4,000 postdoctoral fellows conducting basic, translational, and clinical research
Alumni
Lab Life

August 2024
Left to right, top row: Bhabadeb Chowdhury, Shikha Joon, Mahesh Agarwal
Left to right, bottom row: Alex Compton, Caty Croman, Isaiah Wilt, Scarlett (Guoli) Shi

June 2023
Left to right: Mahesh Agarwal, Isaiah Wilt, Bhabadeb Chowdhury, Abbie Jolley, Isabella Piacentino, Kazi Rahman, Alex Compton, Scarlett (Guoli) Shi

August 2022
Left to right: Mahesh Agarwal, Kazi Rahman, Abbie Jolley, Bhabadeb Chowdhury, Guoli (Scarlett) Shi, Alex Compton

October 2020
Left to right: Kazi Rahman, Alex Compton, Charles Coomer, Saliha Majdoul, Guoli (Scarlett) Shi

July 2019
Left to right: Selena Ding, Alexis Schirling, Saliha Majdoul, Guoli (Scarlett) Shi, Kazi Rahman, Alex Compton
Larger photo

July 2018
Left to right: Tirhas Dempsey, Guoli (Scarlett) Shi, Saliha Majdoul, Kazi Rahman, Alex Compton

July 2017
Left to right: Guoli (Scarlett) Shi, Erin Markle, Saliha Majdoul, Alvin Cao, Kazi Rahman, Alex Compton
Covers

Nature Reviews Immunology, June 2022
Shield's Up! Inhibiting virus entry through cell-intrinsic immunity. Inspired by our review article on entry inhibitors of SARS-CoV-2 and other pathogenic human viruses.

Journal of Molecular Biology, March 2022 (Special Issue)

The EMBO Journal, June 2017
Cover: Zika Virus particles accumulating in infected cells, visualized by electron microscopy. From: Zika virus induces massive cytoplasmic vacuolization and paraptosis‐like death in infected cells.
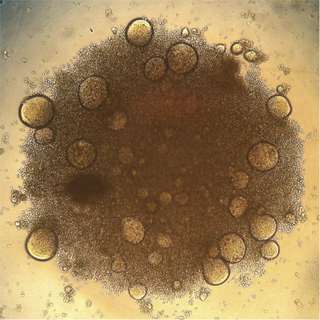
PLOS Pathogens, February 2017
HIV-infected SupT1 T cells forming characteristic multinucleated syncytia following virus-induced cell-cell fusion events in cell culture. Photo taken with an iPhone 4 and a light microscope. Compton et al.
Image Credit: Alex Compton

EMBO Reports, November 2016
Cover: IFITM3 is an antiviral transmembrane protein that inhibits virus entry into host cells. Genomic analysis reveals that IFITM3 is undergoing recurrent gene duplication in primates. IFITM3 homologs exhibit divergent antiviral specificities, suggesting that the duplication and divergence of IFITM genes occurs in response to selective pressure from multiple virus infections.