News and Events
Celebrating CCR Careers: Zhengping Zhuang, M.D., Ph.D.
Zhengping Zhuang, M.D., Ph.D., is a world-renowned expert in experimental pathology, cancer genetics and cancer therapeutics. He has contributed to biotechnological advancements, drug development and clinical translation of his work in tumor biology. After 32 years serving the NCI, he has announced his retirement.
Read MoreResearchers uncover why a leukemia treatment can cause problems with movement and balance
Ara-C, also known as cytarabine, is the most common chemotherapy to cause cerebellar toxicity. Researchers discovered how the treatment leaves certain neurons with double-strand breaks in their DNA.
Read MoreClinical trial researching drug therapy for cancers of the female reproductive organs
A clinical trial led by Jung-Min Lee, M.D., Senior Investigator in the Women’s Malignancies Branch, is researching drug therapy for ovarian, endometrial or cervical cancers.
Read MoreCelebrating CCR Careers: Shioko Kimura, Ph.D.
Shioko Kimura, Ph.D., is a biochemist who specializes in endocrinology. She studies how certain genes and proteins work in the thyroid gland and the lungs. After almost 40 years at NCI, she has announced her retirement.
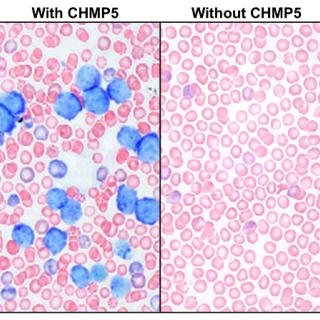
Read MoreProtein shown to promote transcription factors that support cancer genes in leukemia
CCR researchers have discovered that the CHMP5 protein promotes transcription factors that initiate and sustain the expression of cancer genes in T-cell acute lymphoblastic leukemia models.
Read MoreCelebrating CCR Careers: James B. Mitchell, Ph.D.
James (Jim) B. Mitchell, Ph.D., is a radiation biologist whose research focuses on evaluation of agents in combination with radiation that either enhance tumor sensitivity or protect normal tissues. After nearly 50 years at the NCI, Mitchell has announced his retirement.
Read MoreClinical trial researching T-cell therapy for gastrointestinal cancer
A clinical trial led by Nicholas D. Klemen, M.D., Physician-Scientist Early Investigator in the Surgery Branch, is researching T-cell therapy for gastrointestinal cancer.
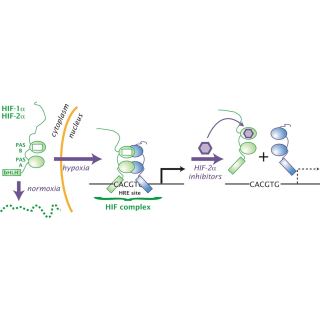
Read MoreBelzutifan works steadily in people with VHL-associated tumors
In a recent clinical trial, belzutifan shows long-lasting benefits for patients with von Hippel-Lindau disease.
Read MoreProtein found on the common cold virus can activate anti-cancer immune response
Researchers found a viral protein that can activate certain immune cells to produce robust anti-cancer responses against liver cancer.
Read MoreClinical trial researching radiation for brain cancer
A clinical trial led by Peter Mathen, M.D., Staff Clinician in the Radiation Oncology Branch, is researching a safe schedule for using radiation for adults with glioblastoma.
Read MoreNew Milestones publication now available
Every year, CCR makes remarkable contributions to the understanding, detection, treatment and prevention of cancer. This digital-only issue of our annual publication, Milestones, features 13 of our top scientific advances from the previous year. These discoveries fall everywhere on the spectrum from basic science to clinical research, ranging from a deeper understanding of the gut-liver axis and how bacteria move, to results and stories from three promising clinical trials. Our researchers have developed AI models to predict when tumors will respond to treatments, built scaffolds of biomaterials to assist with drug delivery, investigated how mitochondria function in healthy and diseased liver cells, and more.
Read More