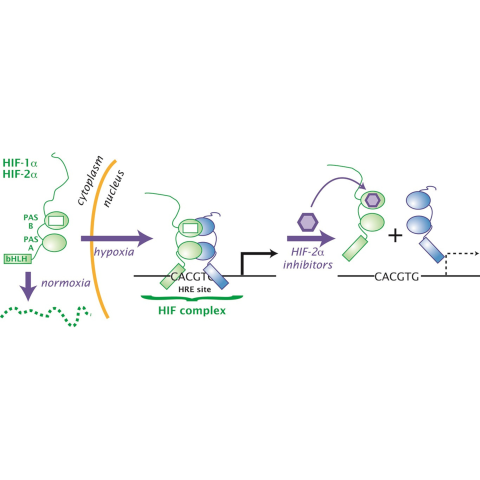
In people with VHL disease, HIF protein complexes accumulate and promote tumor growth. By binding to HIF-2α (green portion of the HIF protein complex), belzutifan (purple hexagon) inhibits HIF’s tumor-promoting activity.
Credit: Richard Bruick, Kevin Gardner, UT Southwestern Medical Center
For people with kidney cancer caused by a rare genetic disorder called von Hippel-Lindau (VHL) disease, treatment with the targeted drug belzutifan (Welireg) can keep their tumors at bay for many years, according to updated results from a clinical trial led by CCR researchers.
After a follow-up period of more than four years, 67% of patients in the trial had experienced at least some notable reduction in the size of one of their tumors.
The drug also shrank tumors that had formed in other common locations in people with VHL-associated kidney cancer, including in the pancreas, brain and spine, and retinas, according to findings published April 11 in Lancet Oncology.
The median time the treatment kept tumors in check was about 40 months, and many patients were able to avoid surgeries to remove tumors.
“VHL is a disease that we manage patients for throughout their entire lives,” said Ramaprasad Srinivasan, M.D., Ph.D., Senior Investigator in the Urologic Oncology Branch and principal investigator of the study.
That’s why it was so important to the research team to follow trial participants closely over a long period, Srinivasan continued. They knew it could shrink tumors in people with VHL-associated cancers and were hopeful the effect would last, he said, “and that’s what we observed.”
The updated results also showed that no new side effects developed over time. “By and large, the drug continues to be very well tolerated by patients,” Srinivasan said. That’s good news because “we need to manage these patients effectively over decades.”
Early results lead to FDA approval
In people with VHL disease, mutations in the VHL gene lead to the accumulation of a protein called HIF-2α, which can fuel the growth of cancerous and noncancerous tumors. Although tumors usually initially form in the kidneys, they often also arise in the pancreas, brain and spine, and the retina.
Most people with VHL disease develop tumors by the age of 30 and continue to develop new tumors throughout their lifetime. Doctors typically perform surgery to remove the tumors, but with each successive surgery the risk of complications increases.
Belzutifan works by blocking the activity of HIF-2α, and the LITESPARK-004 trial was launched to see if it could prevent tumors from forming and growing in people with VHL disease. Initial results from the trial—which was funded by Merck, belzutifan’s manufacturer—showed that after 2 years of treatment, 49% of patients had either a partial or complete response to the drug.
The Food and Drug Administration (FDA) approved belzutifan in August 2021 for the treatment of adults with VHL-associated tumors. But the clinical trial was designed to continue following patients so researchers could get a better understanding of “what happens when we give this drug to people for long periods of time,” Srinivasan said.
Belzutifan gets better with time
The 67% response rate at four years was an improvement over what was seen at two years, which was 49%. The median time it took for patients to have some reductions in the size of their tumors was about 11 months.
Some patients had a complete disappearance of their tumors, with most complete responses (50%) seen in people with pancreatic tumors (a form known as pancreatic neuroendocrine tumors). The number of total surgeries in the trial population dropped from 86 at the time the trial started to 18 afterward.
“You have no idea what that means to these patients,” said W. Marston Linehan, M.D., Chief of the Urologic Oncology Branch, who co-led the study. “We operate on their kidneys, we operate on their brains, on their spine, on their pancreas. To go from 86 surgeries to 18 is just amazing.”
The most common side effects of the treatment included anemia, fatigue, dizziness and nausea. Patients were able to tolerate the side effects, the researchers reported, and after four years, nearly 60% of patients were still on the treatment.
Improving responses with combinations
The researchers pointed out that a higher percentage of patients responding after four years than after two years suggests that belzutifan’s effect on blocking the activity of HIF-2α takes time to translate into visible tumor shrinkage. They are continuing to follow patients to see how effective the drug is with even longer follow-up, such as after 6 or 8 years. The researchers said studies are also needed to understand what happens when patients stop taking the drug for whatever reason.
“We still have a lot to learn,” Srinivasan said, including if and how quickly their tumors come back. “We don't think this drug is going to cure them, because they have an inherited genetic change that predisposes them to [develop] tumors.”
One approach to potentially making the treatment even more effective, he continued, may be to combine belzutifan with other targeted therapies, which is being studied for the treatment of non-hereditary (or sporadic) kidney cancers.
Written by Linda Wang