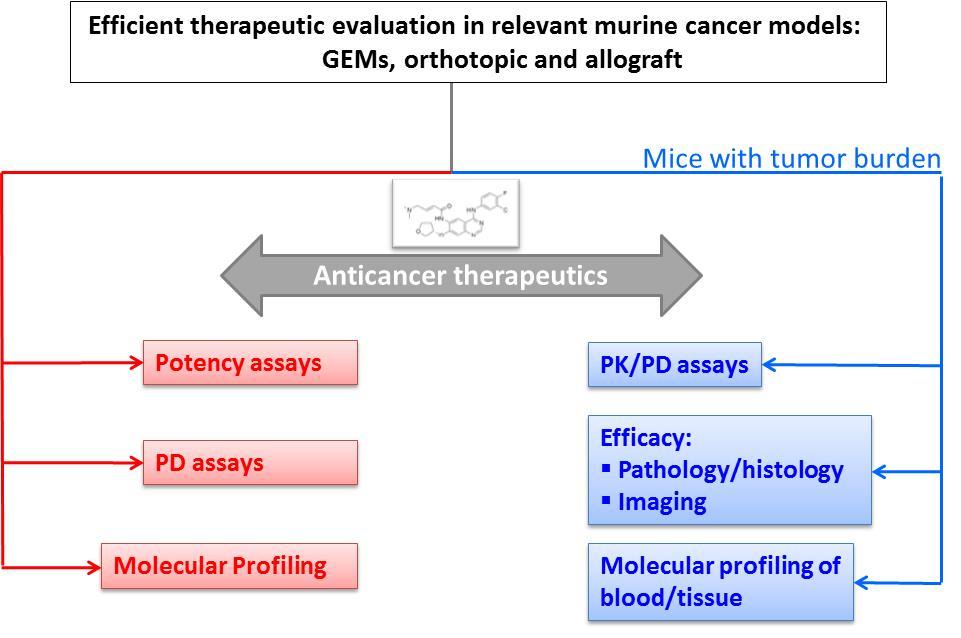
The CAPR Preclinical Evaluation team specializes in evaluation of small molecule drugs and antibodies for cancer therapy and prevention. Their team goal is to reliably produce efficient and effective preclinical evaluation of lead anticancer therapeutics in human-guiding murine cancer models. The team also utilizes gene expression and metabolomics analyses to identify biomarkers of drug treatment effects.
Team Leader
Zoë Weaver Ohler, Ph.D., Principal Scientist
Expertise
- Adaptation of genetically engineered mouse (GEM) models for use in drug treatment studies, including drug combination treatment regimens.
- Adaptation of genetically engineered mouse (GEM) models for use in prevention studies.
- Assessment of pharmacokinetic and pharmacodynamic properties of anticancer therapeutics in murine cancer models.
- Generation of therapy-resistant GEM-derived cancer models.
- Evaluation of preclinical GEM model utility vs. syngeneic orthotopic transplant models in guiding clinical research.
- Imaging endpoint utilization for quantification of drug effects, including one or more modalities such as MRI, PET/CT, ultrasound, and bioluminescence.
- Biomarker and molecular signature discovery for drug treatment effects, including gene expression, immunohistochemistry, and metabolomics evaluation.
- Standard Operating Procedure development and dissemination.
Initiatives
Numerous internal projects and collaborative studies with industry, government and academic partners are ongoing. Current projects include evaluation of targeted therapeutics and biomarkers in mouse models for:
- Non-small cell lung cancer
- Glioblastoma multiforme
- Metastatic melanoma
- Serous epithelial ovarian carcinoma
Team Members
Rajaa El Meskini, Ph.D., Senior Scientist
Devon Atkinson, Research Associate III
Chris McGovern, Research Associate II
Xiuyun (Lucy) Lu, Ph.D., Biologist
Ludmila Szabova, Ph.D., Senior Scientist
Amanda Day, Research Associate II
Deborah Householder, Biologist
Maggie Edouard, Research Associate II
Amy Ries, Research Associate II