Jing Huang, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 37, Room 3140A
- Bethesda, MD 20892-4258
- 240-760-6796
- huangj3@mail.nih.gov
RESEARCH SUMMARY
Dr. Huang’s group explores transcriptional and epigenetic regulation in stem cells and cancer. His earlier research focused on understanding the role of p53 in embryonic stem cells and embryonic development. Currently, the team is dedicated to identifying and targeting transcriptional and epigenetic vulnerabilities in osteosarcoma and breast cancer, aiming to develop innovative therapeutic strategies. To achieve this objective, Dr. Huang's team investigates the functions of several transcription factors (p53, CBFB, and RUNXs) and employs various approaches, including molecular, biochemical, epigenetic, and genomic techniques, as well as utilizing mouse models and human samples.
Areas of Expertise

Jing Huang, Ph.D.
Research
Our research aims to understand how gene expression is regulated in cancer cells and stem cells. In previous studies, we explored the role of p53 in stress responses of embryonic stem cells, uncovering insights into how these cells maintain their genetic stability. Currently, we are investigating transcription factors that drive osteosarcoma and breast cancer, identifying key players like RUNX2, RUNX1, CBFB, and SOX9. Our goal is to use this knowledge to develop new treatments for these cancers in the future.
Project 1: Transcriptional and epigenetic vulnerabilities in osteosarcoma.
Osteosarcoma (OS) stands as the second leading cause of cancer-related death in children and young adults, with its standard care remaining unchanged for the past four decades. The development of new therapies for OS poses significant challenges. Presently, there is a lack of FDA-approved targeted therapies for OS, and immunotherapies, including anti-PD-L1, have proven ineffective in clinical trials. Genomic and epigenomic studies have uncovered the prevalence of RUNX2 and/or MYC amplification in addition to TP53 deletion or mutation in many osteosarcoma (OS) tumors. As these genes encode transcription factors, we posit that transcriptional dysregulation serves as a key factor in driving osteosarcoma progression. Our objective is to elucidate a comprehensive depiction of the core regulatory network in OS.
Our previous research generated insights into the functional interactions between these transcription factors, described as follows.
1) p53 represses RUNX2 via microRNA 34s, a regulation critical for the osteoblast differentiation of mesenchymal stem cells (MSCs). (He et al., Stem Cells, 2015).
2) The CBFB-RUNX2 complex is essential for osteosarcoma cell survival as it recruits the Menin/MLL complex to epigenetically induce MYC expression. (Shin et al., PLOS Genetics, 2016).
3) Several transcription factors determine the lineage choice of OS development from MSCs. For example, SOX9 mediates the development of chondroblastic OS. (He et al, Stem Cell Reports, 2017).
4) SOX9 is a key component of the RUNX2 transcriptional circuitry in OS, acting both a downstream target of RUNX2 and a partner to induce MYC expression. (Kim et al, Cell & Bioscience, 2023).
This early stage of investigation has yielded several molecular targets for OS. For example, we are collaborating with Dr. John Bushweller’s group at UVA to test the effect of CBFB inhibitors in killing osteosarcoma cells.
Our ongoing research builds upon recent discoveries indicating that RUNX2, together with its chromatin-binding partners, regulates immune-related genes. This observation potentially explains why current immunotherapy agents in the clinic lack efficacy in OS. Consequently, we are actively researching the underlying mechanisms of this RUNX2-regulated immunoepigenetics, aiming to develop strategies for enhancing immunotherapy effectiveness in OS.
Project 2: Gene expression dysregulation in breast cancer.
The TP53, CBFB, and RUNX1 genes are frequently mutated in breast cancer, and they encode transcription factors, highlighting the importance of transcriptional/epigenetic dysregulation in breast cancer progression.
Our recent study revealed a functional interaction between p53 and CBFB in breast cancer suppression (Navdeep et al., PLOS Genetics, 2021). One major unexpected theme from our studies is that CBFB plays crucial roles in regulating cytosolic protein translation (Navdeep et al., Nature Communications, 2019) and mitochondrial translation (Navdeep et al., Cancer Research, 2023). Preliminary data suggest that CBFB mutations may serve as a biomarker to predict the efficacy of autophagy inhibitors in breast tumors (Navdeep et al., Autophagy, 2023). Additionally, we have proposed a combination therapeutic strategy for breast tumors bearing CBFB mutations (Navdeep et al., Cancer Research, 2023).
Our ongoing research encompasses two main objectives: 1) elucidating the functions of mutant p53 and its binding partners in transcriptional regulation, and 2) furthering our investigation into the role of CBFB in breast cancer progression. Our aim is to develop new strategies for genomically-guided precision oncology in breast cancer.
Project 3: p53-mediated stress responses of embryonic stem cells.
As embryonic stem cells (ESCs) have the potential to differentiate into many different cell types and are a promising source for cell therapy, maintaining genomic stability and homeostasis is crucial for normal development. Despite this importance, the mechanisms by which ESCs maintain genome stability in response to DNA damage insults were poorly understood. To address this question, we studied how the tumor suppressor protein p53 regulates the DNA damage responses of ESCs. Our research showed that p53 plays an essential role in regulating ESC differentiation after DNA damage by down-regulating the transcription of many ES cell-critical genes (Li et al., Cell Stem Cell, 2015; Zhang et al., Cell Cycle, 2013; Li et al., Molecular Cell, 2012; Lee et al., PNAS, 2010). Recently, we have studied the metabolic stress responses of ESCs and found that Tfcp2l1-mediated fatty acid oxidation plays a critical role in maintaining ESC survival during metabolic stress (Yan et al., EMBO Reports, 2021).
Our research endeavors have generated valuable insights into the mechanisms underlying human embryonic development and developmental disorders.
Methods: We employ a wide range of established and innovative methods to advance our research objectives. Our toolbox includes traditional techniques such as molecular biology methods (e.g., PCR and cloning) and biochemistry techniques (e.g., Western blotting and proteomics). Additionally, we utilize cutting-edge methods such as CRISPR gene editing, advanced genomics techniques (such as ChIP-seq, RNA-seq, and single-cell genomics), mouse genetics, and systems biology approaches. We continuously evaluate and refine our methods, ensuring we have access to the latest technological advancements to tackle complex biological questions. By using these techniques, we aim to gain a deeper understanding of the molecular mechanisms underlying stem cell differentiation and cancer development, ultimately leading to the development of new therapeutic approaches.
Training: Training is a cornerstone of our program, and we offer our trainees a flexible combination of opportunities to develop their skills and knowledge. We provide regular seminars, journal club discussions, and opportunities to publish research findings in high-impact journals. Our goal is to provide a comprehensive training experience that prepares our trainees for successful careers in academia, industry, or government. Many of our past trainees have become independent investigators with their laboratories, and others have gone on to work as scientists in biotech and pharmaceutical companies. We are committed to supporting the success of our trainees and ensuring that they have the skills and knowledge they need to excel in their chosen careers.
Collaborators (former and current): Intramural: Drs. Glenn Merlino, Kent Hunter, Stuart Yuspa, Lalage Wakefield, Beverly Mock, Ji Luo, Mark Simpson, Rosie Kaplan, Daniel Larson, Pamela Robey, Chengyu Liu. Extramural: Drs. Shelley Berger, John Bushweller, Richard Gorlick
Publications
- Bibliography Link
- View Dr. Huang's Complete Bibliography at NCBI.
Dysregulation of mitochondrial translation caused by CBFB deficiency cooperates with mutant PIK3CA and is a vulnerability in breast cancer
The transcription factor CBFB suppresses breast cancer through orchestrating translation and transcription
An Apela RNA-Containing Negative Feedback Loop Regulates p53-Mediated Apoptosis in Embryonic Stem Cells
Biography

Jing Huang, Ph.D.
Dr. Huang earned his Bachelor of Science in Biochemistry from Peking University and completed his Ph.D. in 2004 at the University of Rochester, New York, where he studied estrogen receptor signaling in breast cancer under the mentorship of Drs. Robert Bambara and Mesut Muyan. He further expanded his expertise in cancer epigenetics during postdoctoral training with Dr. Shelley Berger at the Wistar Institute. In 2008, Dr. Huang joined the Laboratory of Cancer Biology and Genetics at the National Cancer Institute (NCI) as a tenure-track Principal Investigator and was granted tenure in 2016. His research accomplishments and leadership have earned multiple distinctions, including the NCI Director’s Innovation Award (co-recipient), the NIH Stem Cell Center grant, the Federal Technology Transfer Award, and the NCI Director’s Mentoring Award. He is also a founding member of the NIH Sarcoma Interest Group.
Job Vacancies
| Position | Degree Required | Contact Name | Contact Email |
|---|---|---|---|
| Postdoctoral Fellow - Epigenetics and Transcription Dysregulation in Cancer | Ph.D. or equivalent, M.D. or equivalent | Jing Huang | huangj3@mail.nih.gov |
Team
News
Calling all sarcoma researchers and enthusiasts—let’s stay connected! Join the NIH Sarcoma Interest Group, a vibrant community dedicated to advancing sarcoma research through collaboration, discussion, and shared insights. Whether you’re working at the bench or bedside, this is your platform to connect with colleagues across NIH and beyond who share your passion. LISTSERV 16.5 - NIH-SARCOMA List at LIST.NIH.GOV
Shasha Wang was invited to present her study at the 2025 CCR-FYI Colloquium.
Hualong Yan was invited to present his work at the 2025 CECB retreat.
Jing Huang is honored to receive the NCI Director's Mentoring Award in 2024 and grateful to his trainees and staff members.
The p53 research in Huang Lab was highlighted in The Scientist
One Protein to Rule Them All | The Scientist Magazine® (the-scientist.com)
Congratulations to Young-Im Kim and Payel Mondal for individually winning the 2025 FARE Award for their studies on molecular and immune targets in osteosarcoma.
Congratulations to Payel, who has been selected to give an oral presentation in the Genetics, Genomics, Chromatin, Signal Transduction and Transcription section of the 2024 CCR-FYI colloquium. She will present her study on transcriptional condensates in osteosarcoma.
Shunlin, our longest-serving member in the laboratory, will retire at the end of 2023. Having served as the lab manager for more than 12 years, we are deeply indebted to him for his dedication and contributions. Congratulations, Shunlin, on achieving another milestone in your life! We will miss you.
Gamze Ayaz was awarded "Best Poster Award" at the 1st LCBG lab retreat in 2023. Congratulations!
Hualong Yan, Young-Im Kim, and Gamze Ayaz were invited to present their osteosarcoma studies at the 2023 Cancer R&D conference in Boston. Congratulations!
Young-Im was honored with the Proteomics Scientific Interest Group Award in 2024 for her study utilizing proteomic techniques to delineate transcriptional networks in osteosarcoma. This award is highly competitive, bestowed upon only one recipient out of approximately 200 who have received FARE awards. She will also be presenting a seminar for the interest group. Congratulations!
We hit the jackpot this year. Congratulations to Young-Im, Gamze, and Shasha for winning three 2024 FARE awards!!!
Congratulations to Gamze and Hualong for co-won the 2023 Federal Technology Transfer Award!
Congratulations to Hualong for receiving the Outstanding Oral Presentation Award for his study on the epigenetic regulation and therapeutic strategies of osteosarcoma! This highly competitive award is granted to only four recipients out of approximately 200 FYI participants.
Hualong and Young-Im have been chosen by the 2023 CCR-FYI Colloquium to deliver oral presentations. This opportunity is given to only 16 fellows out of a pool of about 200 participants. Congratulations to both!
Payel joined the team. Welcome!
Hualong received a 2023 FARE award. Congratulations!
Navdeep will be joining STRM.bio, a startup biotech company in Boston, to lead a team of scientists applying extracellular vesicles in stem cell gene therapy. Congratulations on this remarkable achievement!
Young-Im received a 2022 FARE award. Congratulations!
Yu-Chou will be joining Johnson & Johnson's pharmaceutical arm in 2022 to lead a team of scientists in conducting in vivo testing of chemicals and biologicals for cancer treatment. Congratulations on this significant achievement!
Lab Life

Today, we celebrated Gamze's smooth transition. We will miss her a lot. Fortunately, she will become a senior fellow at NIDDK and continue working at Building 37. Meanwhile, we are celebrating Michelle's summer internship.

We celebrated Shunlin's retirement at Lao Sze Chuan restaurant. It's nice to see Yu-Chou (Jimmy) joining us.

2023 LCBG Christmas party
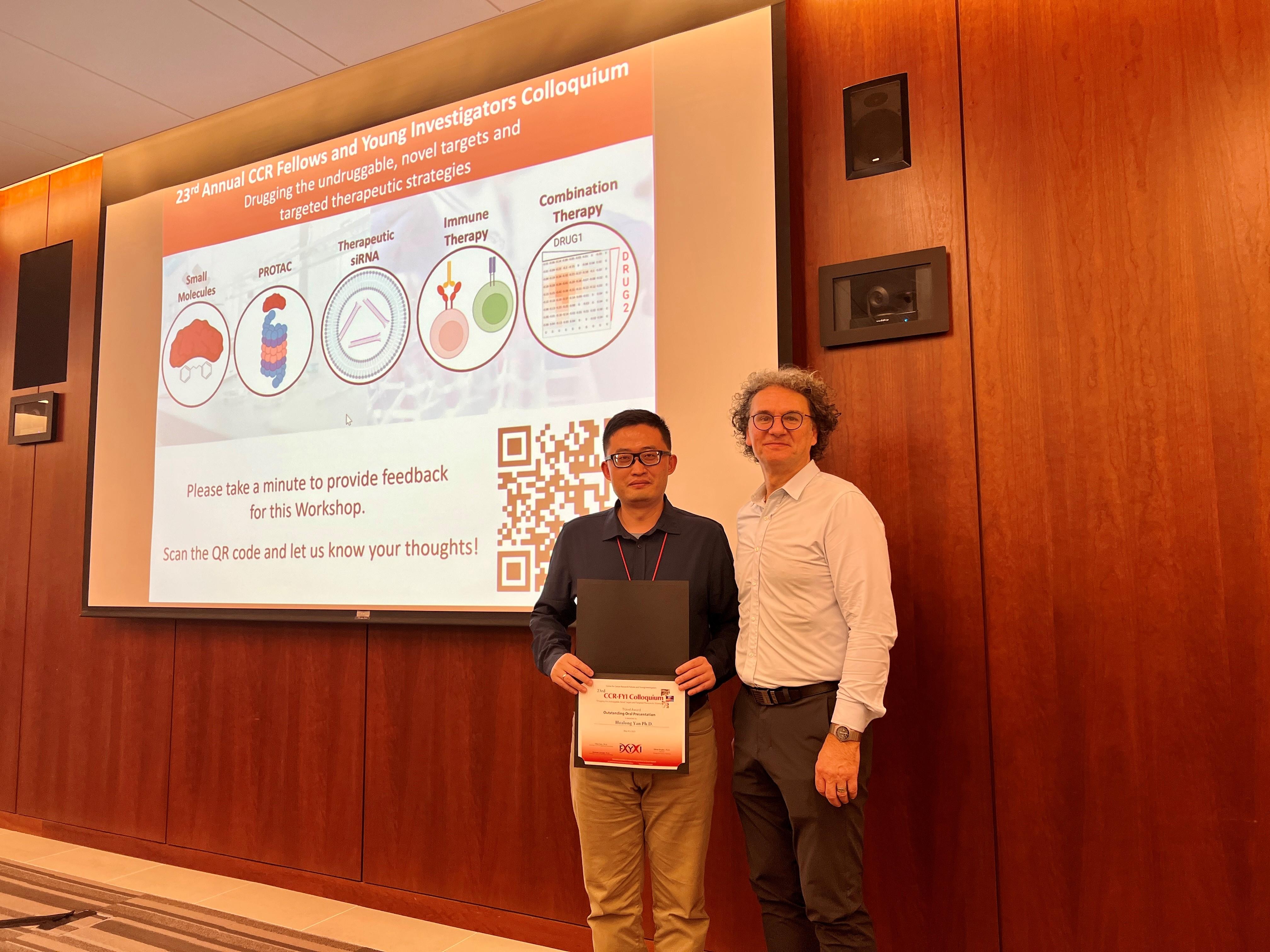
Hualong won an Outstanding Oral Presentation Travel Award at 23rd CCR FYI Colloquium.

Young-Im Kim was selected to present her study of immunotherapy in osteosarcoma at the 23rd CCR FYI Colloquium.

Young-Im Birthday 2022
Navdeep's farewell lunch in 2022. She became a Principal Scientist in STRM.Bio in Boston.

Gamze's birthday celebration 2022
Hualong's birthday 2022