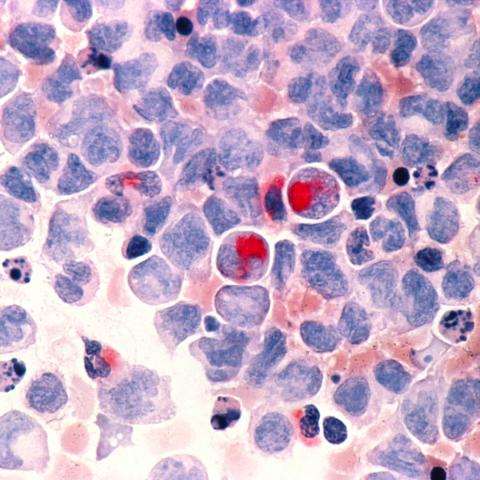
Human cells with acute myelocytic leukemia (AML) in the pericardial fluid, shown with an esterase stain at 400x.
Image source: NCI Visuals Online
Adults with advanced hematologic malignancies may be eligible to participate in a clinical trial at the NIH Clinical Center.
Christopher G. Kanakry, M.D., Lasker Clinical Research Scholar in the Experimental Transplantation and Immunotherapy Branch, is leading a study that may help people with hematologic malignancies. These are cancers that begin in blood-forming tissue, such as the bone marrow, and include leukemia, lymphoma and other blood cancers. Most of these cancers are not curable with chemotherapy alone. In this procedure, a patient receives healthy immune and bone marrow stem cells from a donor. Patients who have had such a transplant are at high risk for graft-versus-host disease (GVHD). This happens when the donated immune cells see the patient’s body as foreign and attack it. Treatment of such patients early after transplant with cyclophosphamide reduces the rate of severe GVHD. However, it has been thought that a very high dose of cyclophosphamide was necessary to kill the immune cells that would attack the body. Dr. Kanakry’s team recently has shown that cyclophosphamide works not by killing these GVHD-causing immune cells, but instead by modifying their response. His team also showed that an intermediate rather than very high dose of cyclophosphamide appears optimal in transplants in preclinical studies and appears effective in preventing severe acute GVHD in an ongoing clinical trial at the NIH Clinical Center. The excess cyclophosphamide causes extra side effects that may be unnecessary, and sicker or older patients may not be able to tolerate such a high dose of cyclophosphamide. The goal of this study is to find out if cutting the cyclophosphamide dose in half can still provide adequate protection against severe acute GVHD while reducing the toxic side effects of transplantation.
Clinicaltrials.gov identifier: NCT04959175
NCI Protocol ID: NCI 000359
Official Title: Phase I/II Study to Reduce Post-transplantation Cyclophosphamide Dosing for Older or Unfit Patients Undergoing Bone Marrow Transplantation for Hematologic Malignancies
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.


