News and Events
Proteins Released from the Nuclei of Dying Cancer Cells Promote Tumor Growth
Material released from dying cancer cells, known as tumor cell nuclear expulsion products (TuNEPs), contains specific proteins that promote the growth of neighboring cancer cells. Targeting these proteins could lead to new treatments that hinder cancer spread and improve patient outcomes.
Read MoreNew synthetic compounds alter RNA expression in bacteria
Researchers have succeeded in developing a number of different compounds that bind to ZTP riboswitches, which regulate RNA, in bacteria. Their approach could one day be used to create a new class of antibacterial drug.
Read MoreA Conversation with Adam Sowalsky, Ph.D.
Adam Sowalsky, Ph.D., is an Investigator in the Laboratory of Genitourinary Cancer Pathogenesis. He is seeking to tease apart the molecular mechanisms underlying prostate cancers that have a high likelihood of progressing and then identifying patients who might benefit from earlier therapy. He discusses what motivated him to pursue a career in cancer biology as well as what lies ahead for his research.
Read MoreClinical trial evaluates radiopharmaceutical as therapy for biochemically recurrent prostate cancer
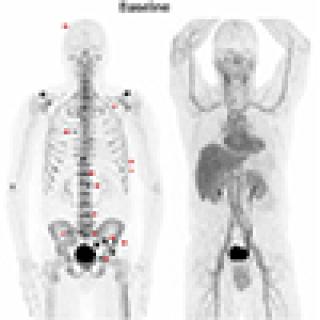
Some men who have been treated for localized prostate cancer (PC) with surgery or radiation still have signs of the disease that are only detected by a blood test (a rising prostate specific antigen or PSA). This is called biochemically recurrent prostate cancer (BCRpc). Investigators in the Center for Cancer Research are leading a clinical trial exploring an option meant to be less toxic for treating BCRpc, which may impact microscopic bone disease seen only on PET scans, using radium-223.
Read MoreImmunotherapy clinical trial tests therapy for metastatic solid tumors
Tumor-infiltrating lymphocytes (TILs) are white blood cells (T cells) that have moved from the blood into a tumor. While tumor cells frequently change their molecular structure to avoid attack by the immune system’s T cells, recent studies by the Center for Cancer Research’s Surgery Branch have found that most TILs don’t recognize newly mutated tumor cells. A clinical trial led by Steve Rosenberg, M.D., Ph.D., engineers T cells to recognize newly altered cancer cells that are then given back to the patient to help the immune system kill cancer cells in solid tumors.
Read MoreTwo patient’s stomachs kept “alive” after removal in novel study to understand stomach cancer
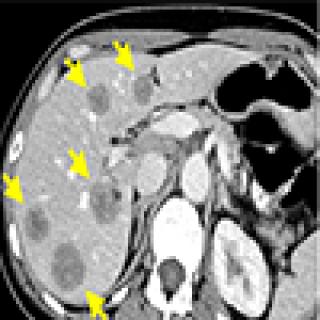
Two women with genetic predisposition to stomach cancer participated in a clinical trial at the Center for Cancer Research where their stomachs were removed and kept “alive” for several days, allowing the researchers to study the development of cancer and the effects of different therapies in unprecedented detail. The goal is to better study stomach cancer under realistic conditions and find novel, effective treatments.
Read MoreJoe Chinquee volunteers with Navajo Nation during COVID-19 pandemic
Joe Chinquee, D.H.Sc., M.B.A., M.T. (A.S.C.P.) D.L.M., Clinical and Scientific Manager of the Laboratory of Pathology, served on the frontlines of the COVID-19 pandemic. In an effort to give back, he was deployed on a month-long mission to assist the U.S. Public Health Service at the Navajo Nation in New Mexico. Chinquee said: “At the NIH, I’ve been given so many opportunities and rewards. I will never stop giving back, but I would not be able to volunteer for these missions without the full support of my NCI leadership.”
Read MoreIn Memoriam: Flossie Wong-Staal, Ph.D.
The Center for Cancer Research mourns the recent death of past colleague Flossie Wong-Staal. She was a major figure in the discovery of HIV and the first to clone the virus.
Read MoreLieutenant and Research Nurse Matt Lindsley shares his experiences and public service role
Matthew Lindsley, MPH, MSN, RN PHNA-BC, is a Lieutenant in the United States Public Health Service. As a Research Nurse Specialist for the NCI’s Center for Cancer Research, Neuro-Oncology Branch, Matt helps patients with brain and spine tumors who come to NIH for treatment. He was recently deployed to aid in the COVID-19 response mission in Washington state for a number of weeks.
Read MoreMary Kearney discusses coronavirus with National Geographic
Mary Kearney, Ph.D., Senior Scientist in the Host-Virus Interaction Branch, was recently quoted in a National Geographic article discussing how long coronavirus lasts inside the body. “Where there’s long-term persistence, there can be long-term consequences,” she says.
Read MoreClinical trial tests combination therapy for glioblastoma multiforme
Glioblastoma multiforme (GBM) is a type of brain cancer where treatments include radiation therapy, chemotherapy and surgery, but survival rates are poor. Investigators are testing an anticancer drug selinexor, which may make GBM cells less resistant to radiation therapy and allow radiation therapy to kill more cancer cells.
Read More