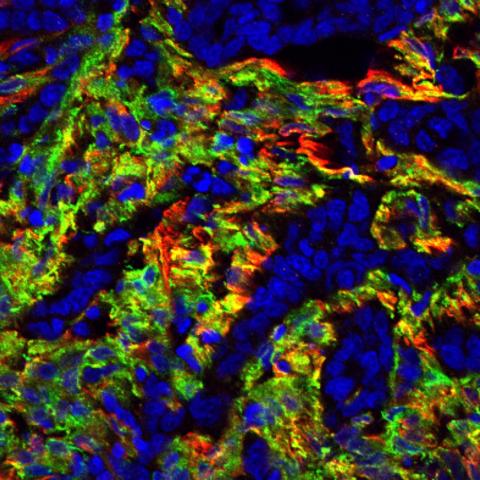
Human colon cancer fluorescently labeled for TEM8 (green) and alpha-smooth muscle actin (red), a marker for a type of activated cancer-associated support cell. Tumor cells are identified by islands of blue nuclei surrounded by the support cells.
Image credit: Brad St. Croix
Researchers at CCR have found that nutrient-deprived solid tumors rely on a steady stream of glutamine, a type of fuel for energy production generated from collagen breakdown in support cells near the tumor. This finding, published November 18, 2022, in Nature Communications, reveals a novel strategy to slow the growth of tumors by blocking collagen uptake — a pathway not targeted by other anti‑cancer drugs.
Collagen is the most abundant protein in the human body. It offers structural support to tissues, impacts tissue growth and repair and is found throughout solid tumors. “If ever there was a likely backup fuel for a tumor that had outgrown its blood supply, I think collagen might make the most sense” suggests Brad St. Croix, Ph.D., Senior Scientist in the Mouse Cancer Genetics Program.
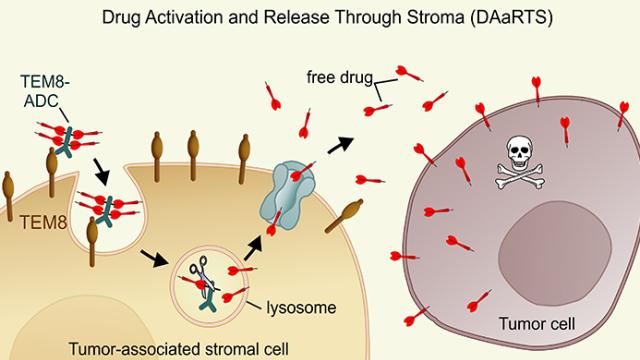
St. Croix’s interests in collagen were piqued when he discovered that a collagen-binding protein, called TEM8/Antxr1, simultaneously played a role in collagen turnover and tumor cell growth. In previous experiments, he found that tumors grew poorly in mice that lacked TEM8 protein. His team wanted to know if collagen turnover was somehow linked to tumor growth.
Using animal and cell co-culture models, Dr. Kuo-Sheng Hsu, lead author of the study, found that tumor support cells use TEM8 to consume and break down collagen into glutamine to feed nearby tumor cells. With a secure supply of glutamine, tumor cells that would normally die from lack of nutrition are able to thrive. “It’s been known for many years that cancer cells are extremely sensitive to glutamine,” explains St. Croix, “but so far nobody knows how to exploit that safely.”
Previous attempts to design glutamine-blocking drugs failed due to severe toxic side effects as glutamine is required throughout the body. St. Croix’s team, however, developed a TEM8-neutralizing antibody that blocked collagen uptake to tumor support cells, halting the glutamine supply only to those cells near the tumor. The antibody drastically decreased tumor growth in animal models with no toxic side effects.
“Now that we understand how TEM8 mediates tumor growth, we have an opportunity to devise complementary cancer-fighting strategies,” St. Croix says. “TEM8-neutralizing antibodies allow us to kill the regions of tumors currently missed or less affected by conventional cancer therapeutics.”
St. Croix foresees an important use for the TEM8 antibody as an anti‑cancer agent that can be used in combination with existing therapies. He is eager to form partnerships to move this drug into the clinic. “The primary goal of our research laboratory is to create better therapies for patients,” says St. Croix.