Our research mission is to develop a comprehensive program aimed at understanding and treating myeloid malignancies in children and adults. Our program has four overarching themes:
- Theme 1: Pre-clinical models to study MDS biology and therapy
- Theme 2: Post-transcriptional regulation in MDS/AML
- Theme 3: Germline predispositions to myeloid malignancy
- Theme 4: Role of the immune system in control of MDS/AML
Learn more about each one in the sections below.
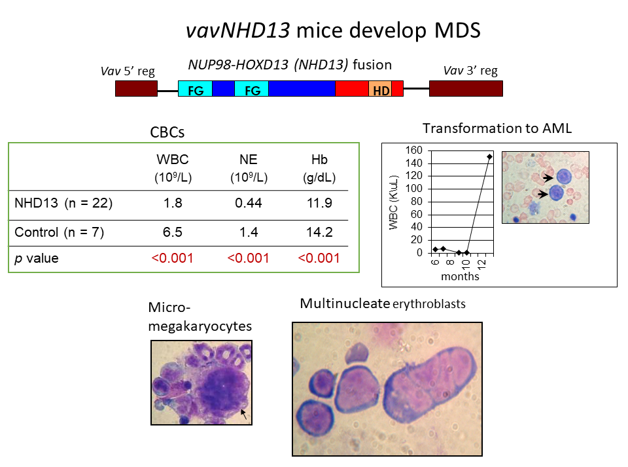
Using the tools of molecular biology and molecular genetics, Dr. Aplan’s lab aims to understand the causes of hematopoietic malignancy, including myelodysplastic syndromes (MDS), acute myelogenous leukemia (AML), T cell acute lymphoblastic leukemia (ALL), and B cell precursor ALL. The general approach taken is to identify mutations, such as chromosomal translocations and single nucleotide variants, that are present in leukemic cells, and study these mutations using a combination of in vitro and in vivo models. Recent accomplishments include generation of the first genetically engineered model for MDS (the NUP98-HOXD13 or NHD13 mouse), a genetically engineered model for progenitor B1 cell ALL, and the discovery of templated sequence insertion polymorphisms (TSIPs) in humans. The NHD13 mouse has been used by numerous investigators in academia and industry to study MDS biology, including the role of the immune system and an inflammatory microenvironment in the development and progression of MDS. Moreover, this model was used to provide proof of concept for the development of luspatercept, the only new drug to receive FDA approval for the treatment of MDS in the past decade. (PI: Peter Aplan)
Additional studies are focused on the MDS/AML microenvironment, and determining how AML cells can influence or “shape” the microenvironment which, in turn, influences the number and function of co-existing normal hematopoietic stem and progenitor cells (HSPCs). Furthermore, there is a growing understanding of the importance of myeloid and stromal cells in the regulation of the bone marrow niche environment during normal development/aging and pathology.
Approaches that rebalance this dysregulated microenvironment may, in combination with other therapies, prevent disease progression or limit relapse. For example, a recent clinical trial of pexidartinib, a CSF1R inhibitor in recurrent, refractory leukemias and solid tumors suggests that approaches that target the immune suppressive microenvironment may be effective. (PI: Rosie Kaplan)
Changes in post-transcriptional regulation are a hallmark of myeloid diseases. For example, >60% of MDS patients have somatic mutations in the core spliceosomal machinery, and spliceosome mutations are enriched in relapsed-refractory AML. Similarly, germline mutations in the translation and ribosome biogenesis machinery selectively result in anemia and other defects in myelopoiesis.
We hypothesize that characterization of post-transcriptional regulation (splicing, translation) in immunological sub-populations, correlated with underlying DNA mutations, will reveal new pathways of disease progression and treatment. For example, we will identify how splicing factor mutations modify the immune microenvironment and how spurious translation might generate neo-antigens, both of which contribute to success/failure of immunotherapy. (PI: Dan Larson)
RNA modifications also play a major role in myelopoiesis and have been identified as synthetic lethalities in AML. The importance of the ‘epitranscriptome’ in normal and healthy hematopoiesis points to the importance of post-transcriptional regulation in this tissue. For example, CCR investigators have discovered and characterized the importance of mRNA acetylation in translational regulation through the enzyme NAT10. We hypothesize that biochemical characterization of mRNA modifications in patient samples with mass spectrometry and sequencing will reveal new pathways for targeted therapeutic intervention. (PI: Shalini Oberdoerffer)
RUNX1 mutations
Autosomal dominant germline RUNX1 mutations are associated with qualitative and quantitative platelet disorders and a 20-50% lifetime risk of hematologic malignancy. Currently, there is insufficient understanding of the variability in penetrance of cancer development across different families and even among individuals within the same family. In addition, the disease manifestations outside of the hematopoietic tissues have not been studied systematically.
We started a comprehensive natural history study on patients with germline RUNX1 mutations in 2019, which has quickly become the largest such study in the world with enrollment of more than 200 patients and family controls from 50 families. We perform comprehensive genomic studies and extensive phenotyping. We have identified previously unrecognized allergic, immunologic, gastrointestinal, and pulmonary symptoms in many of our patients, with consultation of clinical and research faculty from 6 ICs.
The RUNX1 natural history study is shaping up to be an international hub for patient referrals, a central collection of data and patient samples, and a focal point for collaborative clinical and translational studies to understand the disease mechanism, to identify biomarkers for disease progression, and to improve patient monitoring, management, and treatment. (PI: Paul Liu; Medical Director: Lea Cunningham)
GATA2 Deficiency
In 2011, germline mutations in GATA2 were shown to be responsible for a syndrome termed “MonoMAC” at the NIH for lack of monocytes and Mycobacterium avium complex infections. In the interval between the discovery of this syndrome, we have identified nearly 50 different mutations in GATA2 responsible for the syndrome including an enhancer mutation in intron 5, demonstrated second hits in ASXL1 and STAG2 as important variables in disease progression, and carried out allogeneic stem cell transplantation in over 75 patients on protocol 13-C-0132 (NCT01861106). We continue to investigate the important unknowns in GATA2 deficiency, including the factors responsible for progression, and alternative approaches to treatment including CRISPR technologies. (PI: Dennis Hickstein)
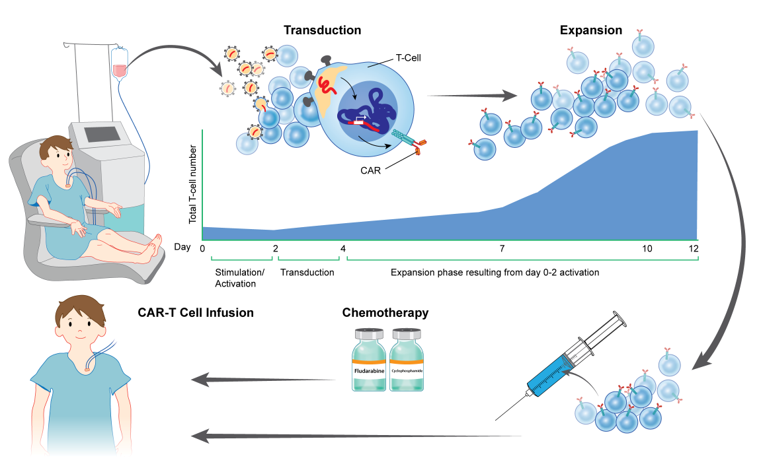
Immunotherapeutic approaches, including (CAR) T-cell based strategies and other antibody-based therapies that target surface proteins found on leukemia cells, may improve outcomes for treating chemotherapy refractory disease and serve as a new paradigm to treat high-risk hematologic malignancies in children, adolescents and young adults. Multiple clinical trials have demonstrated the safety and efficacy of the CD33-targeted antibody-drug conjugate gemtuzumab ozogamicin, and a recent phase 3 trial demonstrated improved event-free survival in pediatric patients with newly diagnosed AML. Based on promising preclinical studies demonstrating robust activity of CD33 antigen-redirected chimeric antigen receptor (CAR) T cells in AML xenograft models the Pediatric Oncology Branch recently began a phase I trial of a new lentiviral CD33 CAR T cell for children and young adults with relapsed/refractory AML. This trial will specifically investigate mechanisms of resistance to CD33 CAR therapy and identifying parameters that are important for response. (PI: Nirali Shah)
Chronic inflammation is central to MDS disease pathology, as evidenced by pro-inflammatory cytokines in the bone marrow milieu, transcriptional upregulation of inflammatory genes, and dysregulation of innate immune signaling pathways. These inflammatory occurrences result in both increased cellular self-renewal as well as increased cell death of MDS hematopoietic stem and progenitors (HSPC) leading to differentiation defects, the fundamental aberration characterizing the disease. Concurrently, chronic inflammation suppresses normal HSPC function and provides an acquired, competitive advantage to the malignant clone fostering disease progression. We find chronic inflammation, and specifically innate immune activation, manifests in the presence of vast genetic diversity in MDS patients and that delineation of the convergence points will drive the development of novel, targeted therapeutic approaches. (PI: Kathy McGraw)