Jeffrey Schlom, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 10, Room 8B09
- Bethesda, MD 20892-1750
- 240-858-3463
- js141c@nih.gov
RESEARCH SUMMARY
As Co-Director of the Center for Immuno-Oncology, Dr. Schlom directs a translational research program in cancer immunology and immunotherapy. He has pioneered the use of novel immunotherapeutics, both as monotherapy and in combination therapies, for a range of human cancers. His studies involve the translation of hypothesis-driven preclinical studies to science-driven clinical trials. Dr. Schlom works closely with clinicians in the CIO and with investigators in multiple Laboratories/Branches of the NCI and NIH, and at extramural Cancer Centers. Dr. Schlom also works closely with investigators in multiple Biotech and Pharma companies, via Cooperative Research and Development Agreements, to rapidly translate preclinical findings into agents for clinical studies. Dr. Schlom’s studies involve the design and development of novel therapeutic cancer vaccines, immunocytokines, checkpoint inhibitor monoclonal antibodies, inhibitors of immune suppressive entities, as well as agents and strategies to modify the tumor microenvironment to render tumor cells more susceptible to immune-mediated therapy.
Areas of Expertise

Jeffrey Schlom, Ph.D.
Research
The CIO Translational Research area functions as a multidisciplinary and interdisciplinary translational research programmatic effort with the goal of developing novel immunotherapies and immunotherapeutic strategies for cancer. Focus is placed on the development of novel immunotherapeutics, not only as monotherapies, but more importantly, in combination with other immune-mediating modalities, and other conventional or experimental therapies, as part of an immuno-oncology programmatic effort. Within this effort are several research groups which interact closely with the clinical groups of the CIO, and with collaborators within the NCI intramural and extramural programs, and with investigators in the private sector. As Co-Director of the CIO, Dr. Jeffrey Schlom directs the Translational Research area of investigation with Dr. James Hodge as Deputy Director for Translational Research.
STRATEGIC PLAN
The CIO Translatoinal Research program takes advantage of the uniqueness of the NCI intramural program in that it spans high-risk basic discovery research in immunology and tumor biology, through preclinical translational research, to paradigm-shifting clinical trials. Focus is placed on the design and development of novel 'off-the-shelf' recombinant immunotherapeutics and immunomodulators that can be used in clinical studies at numerous institutions. This is accomplished in part via Cooperative Research and Development Agreements (CRADAs) with partners in the private sector. The immunotherapeutics and immune modulators that we have developed have also enabled multiple collaborations with clinical investigators at extramural Cancer Centers.
While the use of checkpoint inhibitor monoclonal antibodies (MAb) has shown clear clinical benefit in patients with a spectrum of malignancies, in the vast majority of solid tumors, however, <20% of patients benefit from this class of therapies. The CIO is involved in the design and development of a spectrum of immunotherapeutic and immunomodulatory agents as well as novel strategies employing these agents in preclinical and multiple clinical studies.
The preclinical and clinical immunotherapy studies employing a spectrum of different immunotherapeutic agents including vaccines, checkpoint inhibitors, immune modulators, and inhibitors of immune suppressive entities have the potential to convert tumors that currently do not respond to checkpoint inhibition monotherapy (so-called “cold tumors”) into permissive immunogenic targets leading to clinical benefit in patients with multiple types of tumors. A major emphasis of these studies is to also better understand the mechanisms of both host immune cell activation and resistance of tumors to immunotherapeutic approaches, both within the tumor microenvironment and in the peripheral immunome. These studies may define which patients will respond to combination immunotherapies when interrogated either (a) prior to the initiation of treatment or (b) early in the therapeutic regimen.
AGENTS UNDER PRECLINICAL AND CLINICAL INVESTIGATION AS MONOTHERAPY OR IN COMBINATION THERAPIES ARE:
Recombinant vaccines: (a) Admixtures of proprietary recombinant adenovirus vectors containing transgenes for CEA, MUC1, PSA, and brachyury. The transcription factor brachyury has been identified as a driver of the epithelial-to-mesenchymal transition (EMT) process, stemness, and resistance to therapy. (b) Recombinant poxviral vectors expressing three costimulatory molecules and expressing transgenes for either PSA, CEA plus MUC1, or brachyury. (c) Therapeutic vaccines targeting HPV.
Checkpoint inhibitors: (a) anti-PDL1 (avelumab) and (b) anti-PDL1/TGFβR2 (bintrafusp alfa).
Immune enhancers: (a) IL-15/Ra/Fc immunocytokine (N-803), (b) tumor targeting IL-12 immunocytokine (NHS-IL12), (c) entinostat HDAC inhibitor, and (d) IDO inhibitor.
Costimulators: agonist antibodies to OX40, 41BB, and GITR.
Inhibitors of immune suppressive entities: (a) small molecule IL-8 inhibitor (IL-8 receptor antagonist), and (b) anti-PDL1/TGFβR2 (bintrafusp alfa).
SELECTED RESEARCH ACTIVITIES
The major goals of these studies are (a) to design and develop specific "off-the-shelf" immunotherapeutics for the therapy of human carcinomas, (b) to define mechanisms of action and strategies in preclinical studies and the clinical translation of the use of these immunotherapeutics as part of an immune-oncology platform, and (c) to interrogate so-called "non-immune"–based therapies for their effect on human immune cell subsets and tumor cells to provide the rationale for their combined use in immunotherapeutic regimens.
A NOVEL CHECKPOINT INHIBITOR ANTI-PDL1 MAb
Avelumab is a fully human IgG1 monoclonal antibody (MAb) that binds to PD-L1, inhibiting its binding to PD-1, and also has the ability to mediate antibody-dependent cell-mediated cytotoxicity (ADCC). We interrogated avelumab in preclinical models and then established the safety and pharmacokinetics of avelumab in patients with solid tumors.
Avelumab has demonstrated clinical activity in a range of human cancers and has been approved by the Food and Drug Administration for the therapy of Merkel cell carcinoma and indications in bladder carcinomas.
Since avelumab has been shown in prior in vitro studies to mediate ADCC versus a range of human tumor cells, we designed a study to investigate the effect of avelumab on immune cell subsets in the peripheral blood of cancer patients prior to and following multiple administrations. No statistically significant changes in any of the 123 immune cell subsets analyzed were observed at any dose level, or number of doses, of avelumab. Increases in the ratio of sCD27:sCD40L were observed, suggesting potential immune activation. These studies demonstrated the lack of any significant effect on multiple immune cell subsets, even those expressing PD-L1, following multiple cycles of avelumab.
Natural killer (NK)-92 cells, and their derivative, designated aNK, were obtained from a patient with non-Hodgkin lymphoma. Prior clinical studies employing adoptively transferred irradiated aNK cells have provided evidence of clinical benefit and an acceptable safety profile. aNK cells have now been engineered to express IL-2 and the high affinity (ha) CD16 allele (designated haNK). Prior in vitro studies have shown that avelumab has the ability to mediate ADCC of human tumor cells when combined with NK cells. These studies thus provide the rationale for the clinical evaluation of the combined use of avelumab with that of irradiated adoptively transferred haNK cells or for the use of a recently developed anti-PDL1 haNK bifunctional agent.
A NOVEL BIFUNCTIOAL IMMUNOMODULATOR: BINTRAFUSP ALFA
Tumors evade host immune surveillance through multiple mechanisms, including the generation of a tumor microenvironment that suppresses immune effector function. Secretion of TGFβ and upregulation of immune checkpoint programmed cell death ligand-1 (PD-L1) are two main contributors to immune evasion and tumor progression. We examined the efficacy of a first-in-class bifunctional checkpoint inhibitor, the fusion protein bintrafusp alfa, comprising the extracellular domain of human TGFβRII linked to the C-terminus of human anti-PD-L1 heavy chain (anti-PDL1). We demonstrated that Bintrafusp alfa reduces plasma TGFβ1, binds to PD-L1 in the tumor, and decreases TGFβ-induced signaling in the tumor microenvironment in mice. In murine breast and colon carcinoma models, bintrafusp alfa decreased tumor burden and increased overall survival as compared to targeting TGFβ alone. Bintrafusp alfa treatment also promoted CD8 T cell and NK cell activation, and both of these immune populations were required for optimal mediated tumor control. These findings demonstrate the value of using Bintrafusp alfa to simultaneously target TGFβ and PD-L1/PD-1 immunosuppressive pathways to promote anti-tumor responses and efficacy and support the clinical use of this agent as a monotherapy or in combination with other immunotherapies.
The lack of serial biopsies in patients with a range of carcinomas has been one obstacle in our understanding of the mechanism of action of immuno-oncology agents as well as the elucidation of mechanisms of resistance to these novel therapeutics. While much information can be obtained from studies conducted with syngeneic mouse models, these models have limitations, including that both tumor and immune cells being targeted are murine and that many of the immuno-oncology agents being evaluated are human proteins, and thus multiple administrations are hampered by host xenogeneic responses. Some of these limitations are being overcome by the use of humanized mouse models where human peripheral blood mononuclear cells (PBMC) are engrafted into immunosuppressed mouse strains. By using the NSG-β2m-/- mouse strain humanized with PBMC, we demonstrated (a) the effects of bintrafusp alfa administration on human immune cells in the periphery vs. the tumor microenvironment (TME) using three different human xenograft models; (b) temporal effects upon multiple administrations of bintrafusp alfa; (c) phenotypic changes induced in the TME, and (d) variations observed in the use of multiple different PBMC donors. These results may guide the future use of this agent or similar immunotherapy agents as monotherapies or in combination therapy studies.
Clinical studies of bintrafusp alfa. Nineteen heavily pretreated patients with advanced solid tumors received bintrafusp alfa. The maximum tolerated dose was not reached. Bintrafusp alfa saturated peripheral PD-L1 and sequestered any released plasma TGFβ-1, -2, and -3 throughout the dosing period and was shown to have a manageable safety profile. There were signs of clinical efficacy across all dose levels, including one ongoing confirmed complete response (cervical cancer), two durable confirmed partial responses (PR; pancreatic cancer; anal cancer), one near-PR (cervical cancer), and two cases of prolonged stable disease in patients with growing disease at study entry (pancreatic cancer; carcinoid).
In phase 1 and phase 2 trials, 59 patients with advanced human papillomavirus (HPV)-associated cancers received bintrafusp alfa. The confirmed objective response rate in the checkpoint inhibitor-naive population was 30.5% with five complete responses; eight patients had stable disease for a disease control rate of 35.6%. In addition, three patients experienced a delayed partial response after initial disease progression, for a total clinical response rate of 41.4%. These findings supported further ongoing investigations of bintrafusp alfa in patients with HPV-associated cancers.
A TUMOR-TARGETING IMMUNOCYTOKINE: NHS-IL12
The immunocytokine NHS-muIL12 consists of two molecules of IL-12 fused to NHS76; it is a tumor necrosis-targeting human IgG1.The antitumor activity of systemic administration of NHS-muIL12 was investigated on MB49luc tumors, an aggressive orthotopic bladder cancer model. Studies revealed a significant reduction in bladder tumor burden after administration of NHS-muIL12. Temporal analyses of the bladder tumor microenvironment (TME) initially revealed a large accumulation of myeloid-derived suppressor cells (MDSCs) and macrophages that elicited potent immunosuppression. Immunosuppression was characterized by the inability of CD4+ and CD8+ T cells to respond to broad-based immune stimulants. NHS-muIL12 administration resulted in temporal-dependent reductions in the number of MDSCs, macrophages and tumor-associated TGFβ, which culminated in a re-ignition of CD4+ and CD8+ T cells to elicit potent antitumor responses against MB49luc bladder tumors. These studies provide further rationale for the employment of NHS-IL12 as an immunomodulator and clinical immunotherapeutic.
First-in-human phase I trial of NHS-IL12 was carried out in subjects with metastatic solid tumors. The objectives of the phase I study were to determine the maximum tolerated dose (MTD) and pharmacokinetics of NHS-IL12. The MTD was 16.8 mg/kg. A time-dependent rise in IFNγ and an associated rise in IL10 were observed following NHS-IL12. Of peripheral immune cell subsets evaluated, most noticeable were increases in frequencies of activated and mature natural killer (NK) cells and NKT cells. Based on T-cell receptor sequencing analysis, increases in T-cell receptor diversity and tumor-infiltrating lymphocyte density were observed after treatment where both biopsies and peripheral blood mononuclear cells were available. Although no objective tumor responses were observed, 5 subjects had durable stable disease (range, 6–30+ months). NHS-IL12 was well tolerated. Early clinical immune-related activity warrants further studies, including ongoing combination with immune modulators in both preclinical and clinical studies.
EPIGENETIC MODIFICATION OF TUMOR CELLS AND IMMUNE CELLS
Epigenetic priming of both tumor and natural killer (NK) cells was shown to augment antibody-dependent cellular cytotoxicity (ADCC) elicited by the anti-PD-L1 antibody avelumab against multiple carcinoma cell types. Treatment of a diverse array of human carcinoma cells with either the pan-HDAC inhibitor vorinostat or the class I HDAC inhibitor entinostat significantly enhanced the expression of multiple NK ligands and death receptors resulting in enhanced NK cell‒mediated lysis. Moreover, HDAC inhibition enhanced tumor cell PD-L1 expression both in vitro and in carcinoma xenografts. These data demonstrate that treatment of a diverse array of carcinoma cells with two different classes of HDAC inhibitors results in enhanced NK cell tumor cell lysis and avelumab-mediated ADCC. Furthermore, entinostat treatment of NK cells from healthy donors and PBMCs from cancer patients induced an activated NK cell phenotype, and heightened direct and ADCC-mediated healthy donor NK lysis of multiple carcinoma types. This study thus extends the mechanism and provides a rationale for combining HDAC inhibitors checkpoint blockade to potentially increase patient clinical responses. Multimodal therapies using agents that can affect different arms of the immune system and/or tumor microenvironment (TME) might increase clinical responses. We have demonstrated that entinostat, a class I histone deacetylase inhibitor, enhances the antitumor efficacy of the IL15 superagonist N-803 plus vaccine in triple-negative breast and colon murine carcinoma models. Although N-803 plus vaccine induced peripheral CD8+ T-cell activation and cytokine production, there was no reduction in tumor burden and poor tumor infiltration of CD8+ T cells with minimal levels of granzyme B. Collectively, these data demonstrated that the three-agent combination elicits potent antitumor activity by generating a more inflamed TME and thus form the rationale for the use of this combination of agents for patients harboring poorly or noninflamed solid carcinomas.
THERAPEUTIC VACCINES IN COMBINATION THERAPIES
We have developed recombinant vaccine platforms to target a spectrum of tumor-associated antigens that are currently being employed in clinical studies in combination with immune modulators: (a) Admixtures of proprietary recombinant adenovirus vectors containing transgenes for CEA, MUC1, PSA, and brachyury. The transcription factor brachyury has been identified as a driver of the epithelial-to-mesenchymal transition (EMT) process, stemness, and resistance to therapy. (b) Recombinant poxviral vectors expressing three costimulatory molecules and expressing transgenes for either PSA, CEA plus MUC1, or brachyury. (c) Therapeutic vaccines targeting HPV.
Vaccines targeting tumor neoepitopes have the potential to address qualitative defects; however, additional mechanisms of immune failure may underlie tumor progression. In such cases, patients would benefit from additional immune-oncology agents targeting potential mechanisms of immune failure. We explored the identification of neoepitopes in the MC38 colon carcinoma model by comparison of tumor to normal DNA and tumor RNA sequencing technology, as well as neoepitope delivery by both peptide- and adenovirus-based vaccination strategies. To improve antitumor efficacies, we combined the vaccine with a group of rationally selected immune-oncology agents. We utilized an IL15 superagonist to enhance the development of antigen-specific immunity initiated by the neoepitope vaccine, PD-L1 blockade to reduce tumor immunosuppression, and a tumor-targeted IL12 molecule to facilitate T-cell function within the tumor microenvironment. Analysis of tumor-infiltrating leukocytes demonstrated this multifaceted treatment regimen was required to promote the influx of CD8+ T cells and enhance the expression of transcripts relating to T-cell activation/effector function. Tumor-targeted IL12 resulted in a marked increase in clonality of T-cell repertoire infiltrating the tumor, which when sculpted with the addition of either a peptide or adenoviral neoepitope vaccine promoted efficient tumor clearance. In addition, the neoepitope vaccine induced the spread of immunity to neoepitopes expressed by the tumor but not contained within the vaccine. These results demonstrate the importance of combining neoepitope-targeting vaccines , and in that case any anti-tumor vaccine, with a multifaceted treatment regimen to generate effective antitumor immunity.
HPV THERAPEUTIC VACCINES
While prophylactic human papillomavirus (HPV) vaccines will certainly reduce the incidence of HPV-associated cancers, these malignancies remain a major health issue. PDS0101 is a liposomal-based HPV therapeutic vaccine consisting of the immune activating cationic lipid R-DOTAP and HLA-unrestricted HPV16 peptides that has shown in vivo CD8+ T cell induction and safety in a phase I study. We employed the PDS0101 vaccine with two immune modulators previously characterized in preclinical studies and which are currently in phase II clinical trials. Bintrafusp alfa is a first-in-class bifunctional fusion protein composed of the extracellular domains of the transforming growth factor-β receptor type II (TGFβRII) fused to a human IgG1 monoclonal antibody blocking programmed cell death protein-1 ligand (PDL1), designed both as a checkpoint inhibitor and to bring the TGFβRII “trap” to the tumor microenvironment (TME). NHS-interleukin-12 (NHS-IL12) is a tumor targeting immunocytokine designed to bring IL-12 to the TME and thus enhance the inflammatory Th1 response. As a monotherapy, the PDS0101 vaccine generated HPV-specific T cells and antitumor activity in mice bearing HPV-expressing oropharyngeal and lung carcinomas. When all three agents were used in combination, maximum antitumor effects were observed, which correlated with increases in T cells and T-cell clonality in the TME. These studies provided the rationale for the clinical evaluation of combinations of agents that can (1) induce tumor-associated T-cell responses, (2) potentiate immune responses in the TME and (3) reduce immunosuppressive entities in the TME.
The development of a therapeutic vaccine will require the generation of T-cell responses directed against early HPV proteins (E6/E7) expressed in HPV-infected tumor cells. No HPV therapeutic vaccine has been approved by the Food and Drug Administration to date. One method of enhancing the potential efficacy of a therapeutic vaccine is the generation of agonist epitopes. We reported the first description of enhancer cytotoxic T lymphocyte agonist epitopes for HPV E6 and E7. An in silico algorithm revealed six epitopes with potentially improved binding to HLA-A2–Class I, 5/6 demonstrated enhanced binding to HLA-Class I in cell-based assays and 3/6 had a greater ability to activate HPV-specific T cells that could lyse tumor cells expressing native HPV, compared to their native epitope counterparts. These agonist epitopes have potential for use in a range of HPV therapeutic vaccine platforms and for use in HPV-specific adoptive T- or natural killer–cell platforms.
PRGN-2009 is a therapeutic gorilla adenovirus HPV vaccine containing multiple cytotoxic T-cell epitopes of the viral oncoproteins HPV 16/18 E6 and E7, including T-cell enhancer agonist epitopes. PRGN-2009 treatment reduced tumor volume and increased CD8+ and CD4+ T cells in the TME of humanized mice bearing a human cervical tumor xenograft. PRGN-2009 monotherapy in the syngeneic TC-1 model also reduced tumor volumes, generated high levels of HPV16 E6–specific T cells, and increased multifunctional CD8+ and CD4+ T cells in the TME. These studies provided the first evaluation to our knowledge of a therapeutic recombinant gorilla adenovirus vaccine. The promising preclinical antitumor efficacy and induction of HPV-specific T cells provided the rationale for its evaluation in ongoing clinical studies.
A BIFUNCTIONAL ANTI-PDL1/IL-15 SUPERAGONIST
N-809 is a first-in-class bifunctional agent comprising the interleukin (IL)-15 superagonist N-803 fused to two anti-PDL1 domains. Thus, N-809 can potentially stimulate effector immune cells through IL-15 and block immunosuppressive PD-L1. We examined the antitumor efficacy and immunomodulatory effects of N-809 versus N-803+anti-PDL1 combination. We demonstrated that N-809 blocks PD-L1 and induces IL-15–dependent immune effects. N-809 reduced 4T1 lung metastasis, decreased MC38 tumor burden and increased survival versus N-803+anti-PDL1. Compared with N-803+anti-PDL1, N-809 enhanced natural killer (NK) and CD8+ T-cell activation and function in the draining lymph node and tumor microenvironment (TME), relating to increased gene expression associated with interferon and cytokine signaling, lymphoid compartment, costimulation and cytotoxicity. The higher number of TME CD8+ T cells was attributed to enhanced infiltration, not in situ expansion. Increased TME NK and CD8+ T-cell numbers correlated with augmented chemokine ligands and receptors. Moreover, in contrast to N-803+anti-PDL1, N-809 reduced immunosuppressive regulatory T cells, monocytic myeloid-derived suppressor cells and M2-like macrophages in the TME. N-809 thus functions by a novel immune mechanism to promote antitumor efficacy.
SELECTED RECENT PUBLICATIONS
- Minnar CM, Chariou PL, Horn LA, Hicks KC, Palena C, Gameiro SR, Schlom J. Tumor-targeted interleukin-12 synergizes with entinostat to overcome PD-1/PD-L1 blockade-resistant tumors harboring MHC-I and APM deficiencies. J ImmunoTher Cancer (in press).
- Tsai Y-T, Strauss J, Toney NJ, Jochems C, Gulley JL, Donahue RN, Schlom J. Immune correlates of clinical parameters in patients with HPV-associated malignancies treated with bintrafusp alfa. J ImmunoTher Cancer. 10(4):e004601, 2022.
- Hong Y...Gulley JL, Schlom J, Gameiro SR, Sievers C, Allen CT. Cure of syngeneic carcinomas with targeted IL-12 through obligate reprogramming of lymphoid and myeloid immunity. JCI Insight. 2(5):e157448, 2022.
- Gameiro SR, Strauss J, Gulley JL, Schlom J. Preclinical and clinical studies of bintrafusp alfa, a novel bifunctional anti-PDL1/TGFβRII agent: current status. [minireview] Exp Biol Med. doi: 10.1177/15353702221089910. Epub ahead of print, 4/27/22.
- Horn L, Chariou PL, Gameiro SR, Qin H, Iida M, Fousek K...Schlom J, Palena C. Remodeling of the myeloid and extracellular matrix compartments via blockade of LAIR-1 and TGF-beta enables PD-L1-mediated tumor control. J Clin Invest. 132(8):e155148, 2022.
- Mullenix C…Tsai Y-T, Donahue RN...Schlom J, Gulley JL, Rajan A. Joint-predominant rheumatic complications of immune checkpoint inhibitor therapy in patients with thymic epithelial tumors. https://pubmed.ncbi.nlm.nih.gov/34895481/
- Bilusic M, Toney N, Donahue R...Gulley J, Schlom…Geynisman D. A randomized phase 2 study of bicalutamide with or without metformin for biochemical recurrence in overweight or obese prostate cancer patients (BIMET-1). Prostate Cancer Prostatic Dis. pub. online 1/25/22.
- Redman JM, Tsai Y-T...Donahue RN...Jochems C...Schlom J, Gulley JL, Strauss J. A randomized phase II trial of mFOLFOX6 and bevacizumab alone or with combination immunotherapy with AdCEA vaccine plus avelumab in patients with previously untreated metastatic colorectal cancer. The Oncologist. 27(3):198-209, 2022.
- Mohammed A…Disis ML, Jaffee EM…Schlom J…Miller MS. Translational Advances in Cancer Prevention Agent Development (TACPAD) Virtual Workshop on Immuno-modulatory Agents: [Meeting] Report. J Cancer Prev. 26(4):309-317, 2021.
- Gulley JL, Schlom J... Moustakas A. Dual inhibition of TGF-β and PD-L1 as a novel cancer treatment approach [review]. Molec Oncol. doi: 10.1002/1878-0261.13146 Epub ahead of print 12/1/21.
- Robbins Y...Donahue R, Schlom J...Hinrichs C...Gulley JL, Norberg S. Dual PD-L1 and TGF-beta blockade in patiets with current respiratory papillomatosis. J Immunother Cancer. 9(8):e0031132021, 2021.
- Hicks KC, Chariou P, Ozawa Y, Minnar C, Knudson KM, Meyer T, Bian J, Cam M, Gameiro SR, Schlom J. Tumour-targeted interleukin-12 and entinostat combination therapy improves cancer survival by reprogramming the tumour immune cell landscape. Nat Commun. 12(1):5151, 2021.
- DeMaria PJ...Donahue RN, Schwab A, Palena C, Jochems C, Floudas CS, Strauss J, Marté JL, Redman JM...Schlom J, Gulley JL, Bilusic M. Phase 1 open-label trial of intravenous administration of MVA-BN-brachyury vaccine in patients with advanced cancer. J ImmunoTher Cancer. 9(9):e003238, 2021.
- Lee MY...Jochems C, Schlom J, Allen CT. Pre-clinical study of a novel therapeutic vaccine for recurrent respiratory papillomatosis. NJP Vaccines. 6(1):86, 2021.
- Wolfson B, Padget MR, Schlom J, Hodge JW. Exploiting off-target effects of estrogen deprivation to sensitize estrogen receptor negative breast cancer to immune killing. J ImmunoTher Cancer. 9(7):e002258, 2021.
- Reimann H...Schlom J, Gulley JL, Lee KL, Hamilton D, Soon-Shiong P, Fasching PA, Kremer AN. Identification and validation of expressed HLA-binding breast cancer neoepitopes for potential use in individualized cancer therapy. J ImmunoTher Cancer. 9(6):e002605, 2021.
- Ozawa Y...Gameiro SR, Schlom J. Analysis of the tumor microenvironment and anti-tumor efficacy of subcutaneous vs systemic delivery of the bifunctional agent bintrafusp alfa. OncoImmunology. 10(1):e1915561, 2021.
- Bilusic M...Schlom J, Gulley JL. Phase 1 study of a multi-targeted recombinant Ad5 PSA/MUC-1/brachyury-based immunotherapy vaccine in patients with metastatic castration-resistant prostate cancer (mCRPC). J ImmunoTher Cancer. 9(3):e002374, 2021.
-
Horn LA, Fousek K, Hamilton DH, Hodge JW, Zebala JA, Maeda DY, Schlom J, Palena C. Vaccine increases the diversity and activation of intratumoral T cells in the context of combination immunotherapy. Cancers [special issue: Cancer Immunology]. 13:968, 2021.
- Pastor DM and Schlom J. Immunology of Lynch syndrome [review]. Curr Oncol Rep. 23(8):96, 2021.
- Pellom ST...Jochems C, Schlom J. Characterization of a recombinant gorilla-adenovirus HPV therapeutic vaccine (PRGN-2009). JCI Insight. 6(7):141912, 2021.
- Donahue RN...Gulley JL, Schlom J. Interrogation of the cellular immunome of cancer patients with regard to the COVID-19 pandemic. J Immunother Cancer. 9:e002087, 2021.
- Schlom J and Donahue RN. The importance of cellular immunity in the development of vaccines and therapeutics for COVID-19 (Perspective). J Infect Dis. 222(9):1435-1438, 2020.
- Strauss J...Schlom J, Gulley JL. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus–associated malignancies. J Immunother Cancer. 8(2):e001395, 2020.
- Smalley Rumfield C...Jochems C, Schlom J. Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J Immunother Cancer. 8(1):e000612, 2020.
- Morillon II YM...Greiner JW, Schlom J. The development of next-generation PBMC humanized mice for preclinical investigation of cancer immunotherapeutic agents. Anticancer Res. 40: 5329-5341, 2020.
- Knudson KM…Gameiro SR, Schlom J. Functional and mechanistic advantage of the use of a bifunctional anti-PD-L1/IL-15 superagonist. J Immunother Cancer. 8(1):e000493, 2020.
- Hicks KC…Gameiro SR, Schlom J. Cooperative immune-mediated mechanisms of the HDAC inhibitor entinostat, an IL-15 superagonist, and a cancer vaccine effectively synergize as a novel cancer therapy. Clin Cancer Res. 26:704-716, 2020.
- Gatti-Mays ME...Gulley JL, Schlom J, Strauss J. A phase I trial using a multitargeted recombinant adenovirus 5 (CEA/MUC1/Brachyury)-based immunotherapy vaccine regimen in patients with advanced cancer. The Oncologist. 25(6):479-e899, 2020.
- Lee KL…Hamilton DH, Schlom J. Efficient tumor clearance and diversified immunity through neoepitope vaccines and combinatorial immunotherapy. Cancer Immunol Res. 7:1359-1370, 2019.
- Morillon YM...Greiner JW, Schlom J. Temporal changes within the (bladder) tumor microenvironment that accompany the therapeutic effects of the immunocytokine NHS-IL12. J ImmunoTher Cancer. 7(1):150, 2019.
- Gatti-Mays ME…Schlom J, Gulley JL. A phase 1 dose-escalation trial of BN-CV301, a recombinant poxviral vaccine targeting MUC-1 and CEA with costimulatory molecules. Clin Cancer Res. 25(16):4933-4944, 2019.
- Jochems C…Schlom J. The multi-functionality of N-809, a novel fusion protein encompassing anti-PD-L1 and the IL-15 superagonist fusion complex. OncoImmunology. 8(2);e1532764, 2018.
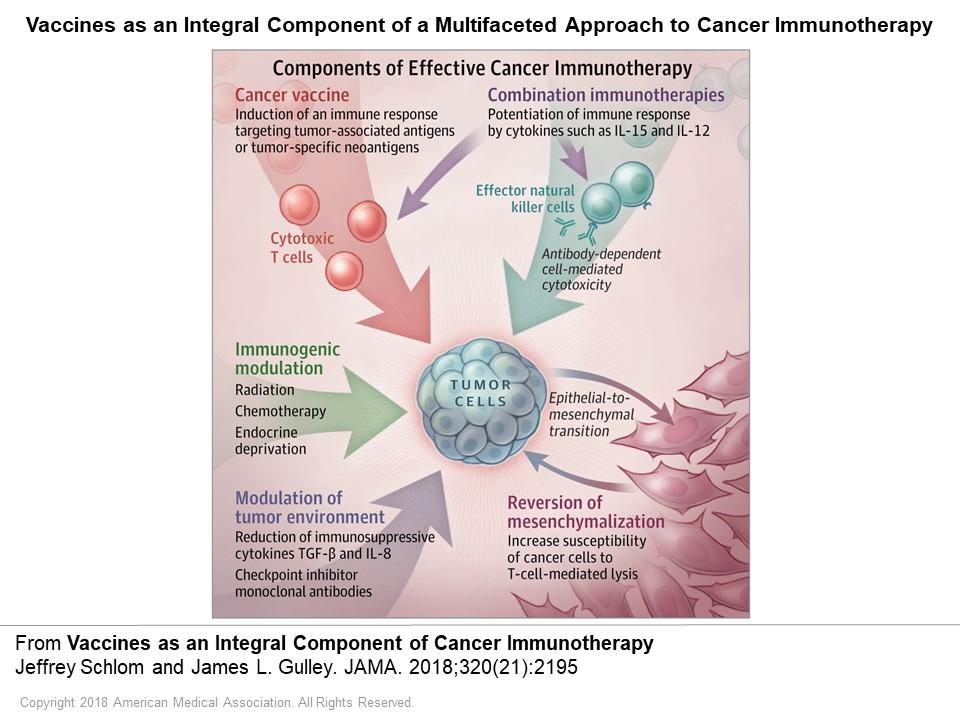
- Schlom J and Gulley JL. Vaccines as an integral component of cancer immunotherapy (Viewpoint). JAMA. 320(21):2195-2196, 2018.
- Strauss J…Schlom J, Gulley JL. First-in-human phase I trial of a tumor-targeted cytokine (NHS-IL12) in subjects with metastatic solid tumors. Clin Cancer Res. 25(1):99-109, 2019.
- Hicks KC…Schlom J. Epigenetic priming of both tumor and NK cells augments antibody-dependent cellular cytotoxicity elicited by the anti-PD-L1 antibody avelumab against multiple carcinoma cell types. OncoImmunology. 7(11):e1466018, 2018.
- Knudson KM...Gameiro SR, Schlom J. M7824, a novel bifunctional anti-PD-L1/TGFβ Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. OncoImmunology. 7(5):e1426519, 2018.
- Strauss J..Schlom J...Gulley JL. Phase 1 trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in advanced solid tumors. Clin Cancer Res. 24(6):1287-1295, 2018.
- Grenga I…Schlom J. Anti-PD-L1/TGFβR2 (M7824) fusion protein induces immunogenic modulation of human urothelial carcinoma cell lines, rendering them more susceptible to immune-mediated recognition and lysis. Urol Oncol. 36(3):93.e1-93.e11, 2018.
- Jochems C...Gulley JL, Schlom J. Analyses of functions of an anti-PD-L1/TGFβR2 bispecific fusion protein (M7824). Oncotarget. 8:75217-75231, 2017.
- Jochems C…Schlom J. ADCC employing an NK cell line (haNK) expressing the high affinity CD16 allele with avelumab, an anti-PD-L1 antibody. Int J Cancer. 141:583-593, 2017.
- Tsang KY...Schlom J. Identification and characterization of enhancer agonist human cytotoxic T-cell epitopes of the human papillomavirus type 16 (HPV16) E6/E7. Vaccine. 35:2605-2611, 2017.
- Donahue RN...Gulley JL, Schlom J. Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti-PD-L1 monoclonal antibody. J ImmunoTher Cancer 5:20, 2017.
- Heery CR…Schlom J, Gulley JL. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 18:587-598, 2017.
CLINICAL TRIALS INVOLVING DR. SCHLOM’S COLLABORATIVE ACTIVITIES:
- Phase II Trial of M7824 in Subjects with HPV Positive Malignancies (PI, Strauss)
- First In-Human Phase I Trial of NHS-IL12 in Subjects with Metastatic Solid Tumors (PI, Gulley)
- A Pilot Study to Investigate the Safety and Clinical Activity of Avelumab (MSB0010718C) in Thymoma and Thymic Carcinoma after Progression on Platinum-Based Chemotherapy (PI, Rajan)
- Phase I/II Trial of Combination Immunotherapy in Subjects with Advanced HPV Associated Malignancies (PDS) (PI, Strauss)
- QUICC: Phase II Trial of Combination Immunotherapy in Subjects with Advanced Small Bowel and Colorectal Cancers (PI, Strauss)
- Docetaxel and PROSTVAC for Metastatic Castration Sensitive Prostate Cancer (PI, Madan)
- Phase II Trial of Combination Immunotherapy in Biochemically Recurrent Prostate Cancer (PI, Madan)
- A Phase I/II Study of Immunotherapy Combination BN-Brachyury Vaccine, M7824, ALT-803 and Epacadostat (QuEST1) (PI, Gulley)
- Phase 1 Open Label Trial of Intravenous Administration of MVA-BN-Brachyury Vaccine in Patients with Advanced Cancer (PI, Bilusic)
- A Sequential Window of Opportunity Trial of Anti-PD-L1/TGF-ß trap (M7824) Alone and in Combination with TriAd Vaccine, and N-803 for Resectable Head and Neck Squamous Cell Carcinoma not associated with Human Papillomavirus Infection (Co-PIs, Redman/Allen)
- Phase I/II of NHS-IL12 Monotherapy and in Combination with M7824 in Advanced Kaposi Sarcoma (Co-PIs, Yarchoan/Ramaswami)
- A Phase II, Open-Label Trial of Bintrafusp Alfa (M7824) in Subjects with Thymoma and Thymic Carcinoma (PI, Rajan)
- A Phase 1/2, Open Label, Dose-Escalation, Safety and Tolerability Study of NC410 in Subjects with Advanced or Metastatic Solid Tumors (Co-PIs, Madan and Pastor)
- WOOT: Phase I/II Trial of HPV Vaccine PRGN-2009 Alone or in Combination with Anti-PD-L1/TGF-β trap (M7824) in Subjects with HPV Positive Cancers (Co-PIs, Strauss/Allen)
- STAT: Phase I/II Trial Investigating the Safety, Tolerability, Pharmacokinetics, Immune and Clinical Activity of SX-682 in Combination with Bintrafusp Alfa (M7824 or TGF-b “trap”/PD-L1) with BN-CV301 TRICOM in Advanced Solid Tumors (STAT) (PI, Gulley)
- A Phase II Trial of Perioperative CV301 Vaccination in Combination with Nivolumab and Systemic Chemotherapy for Resectable Hepatic-Limited Metastatic Colorectal Cancer (PI, Carpizo, Rutgers, multi-center)
- BEST: Phase I/II Trial of the Combination of Bintrafusp Alfa (M7824), Entinostat and NHS-IL12 (M9241) in Patients with Advanced Cancer (PI, Abdul Sater)
Publications
- Bibliography Link
- View Dr. Schlom's PubMed Summary.
Biography

Jeffrey Schlom, Ph.D.
strong>Dr. Jeffrey Schlom is Co-Director of the Center for Immuno-Oncology, Center for Cancer Research, National Cancer Institute, National Institutes of Health. He received his Ph.D. from the Waksman Institute at Rutgers University. Dr. Schlom was formerly Chief of the Laboratory of Tumor Immunology and Biology, CCR, NCI, NIH. Dr. Schlom directs a translational research program in which the latest advances in immunology and immunotherapy are used to design and develop a range of potential novel immunotherapeutic approaches for a variety of human cancers. His most recent work involves the development of novel therapeutic cancer vaccines, checkpoint inhibitors and immune modulators, both as a monotherapy and in combination therapies. The program focuses on the design and development of novel "off the shelf" immunotherapeutics that can be translated from hypothesis-driven preclinical studies to science-based clinical studies both at the NIH and at numerous Cancer Centers throughout the U.S. Dr. Schlom serves on the editorial boards of numerous scientific journals. He has authored more than 800 scientific publications and holds numerous patents for monoclonal antibody and recombinant vaccine generation and uses.
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
Novel T cell stimulation technique drives potent anti-tumor response
STAR0602, a bifunctional therapeutic molecule, selectively activates a subset of T cells through both the TCR and IL-2 receptor signaling pathways. Immunological and antitumor effects in mice were recapitulated in studies of STAR0602 in nonhuman primates and human ex vivo models, wherein STAR0602 boosted human antigen-specific T cell responses and killing of tumor organoids. Thus, STAR0602 represents a distinct class of T cell–activating molecules with the potential to deliver enhanced antitumor activity in checkpoint inhibitor–refractory settings. A phase 1/2 first-in-human, open-label, dose escalation and expansion study of STAR0602 in patients with advanced metastatic cancer has recently commenced. Sci Transl Med. 2023;15(724):eadi0258. doi: 10.1126/scitranslmed.adi0258
Clinical trial researches bone marrow transplants for blood cancers. A clinical trial led by Christopher G. Kanakry, M.D., Lasker Clinical Research Scholar in the Center for Immuno-Oncology, is researching bone marrow transplants [and whether the lymphocytes they contain can help reduce relapse risk] for some types of blood cancers, such as high-risk leukemia, lymphoma, myelodysplastic syndrome or multiple myeloma.
Tumor-targeted interleukin-12 synergizes with entinostat to overcome PD-1/PD-L1 blockade-resistant tumors harboring MHC-I and APM deficiencies. Tumor-intrinsic loss of major histocompatibility complex class I (MHC- I) and aberrations in antigen processing machinery (APM) and interferon gamma (IFN-γ) pathways have been shown to play an important role in immune checkpoint blockade resistance. We demonstrated that the combination of entinostat and NHS- IL12 therapy elicits potent antitumor activity across αPD- 1/αPD- L1 refractory tumors. Our findings provide a rationale for combining the tumor-targeting NHS- IL12 with the histone deacetylase inhibitor entinostat in the clinical setting for patients unresponsive to αPD- 1/αPD- L1 and/or with innate deficiencies in tumor MHC- I, APM expression, and IFN-γ signaling. Minnar et al. Journal for ImmunoTherapy of Cancer 10(6):e004561, 2022.
Tumor-targeted interleukin-12 and entinostat combination therapy improves cancer survival by reprogramming the tumor immune cell landscape. Poorly inflamed carcinomas do not respond well to immune checkpoint blockade. Converting the tumor microenvironment into a functionally inflamed immune hub would extend the clinical benefit of immune therapy to a larger proportion of cancer patients. We have shown that Entinostat, a class I histone deacetylase inhibitor, facilitates accumulation of the necrosis-targeted recombinant murine immune-cytokine, NHS-rmIL12, in poorly immunogenic tumors. This combination therapy reprograms the tumor innate and adaptive immune milieu to an inflamed landscape. Collectively, these findings provide a rationale for combining NHS-IL12 with Entinostat in the clinical setting. Hicks et al. Nature Communications 12(1):5151; 2021.
In Viewpoint: Evolving Issues in Oncology: “Vaccines as an integral component of cancer immunotherapy,” Drs. Jeffrey Schlom and James Gulley describe the opportunities and challenges for vaccine therapies to treat cancers. JAMA. 2018 Dec 4;320(21):2195-2196.
Immunotherapy drug with two targets shows promise against HPV-related cancers. In a Phase 1 clinical trial at the NCI of 43 patients with advanced cancers, the investigational immunotherapy drug bintrafusp alfa shrank the tumors of some patients with advanced human papillomavirus (HPV)-related cancers. “This drug is a promising agent for patients with HPV-related cancers and may potentially benefit these patients more than traditional checkpoint therapies,” said the trial's Principal Investigator, Julius Strauss, M.D., Laboratory of Tumor Immunology and Biology, CCR, NCI.
https://www.cancer.gov/news-events/cancer-currents-blog/2019/immunotherapy-y-trap-hpv-cancers
Clinical trial tests immunotherapy combination for advanced HPV-associated cancers. Dr. Julius Strauss is leading a study using a combination of 3 immunotherapy drugs to treat HPV+ cancers. [NCT04287868]
New trial evaluates immunotherapy combinations in adults with advanced small bowel and colorectal cancers is being led by Dr. Julius Strauss. [NCT04491955]
Clinical trial will test triple-drug combination against aggressive colon and HPV-associated cancers. Dr. Julius Strauss discusses promising immunotherapy drug for patients with HPV-related cancers. [NCT04708470]
Seminars
The CIO Distinguished Lecture Series is a premier virtual seminar series aimed at showcasing groundbreaking advancements in immunotherapy for cancer research. Recent highlights include clinical successes in CAR-T cell therapies targeting solid tumors, translational breakthroughs in bispecific T-cell engagers (BiTEs) for enhanced tumor specificity, and bench research on novel immune checkpoint inhibitor combinations that overcome key resistance mechanisms. The series also explores the development of personalized neoantigen vaccines and strategies for modulating the tumor microenvironment to boost immune response. By fostering a collaborative environment, the series disseminates cutting-edge research findings across clinical, translational, and bench research, bridging the translation of novel immunotherapeutic strategies into clinical practice and ultimately advancing our understanding and treatment of various cancers. The audience are scientists and clinical researchers within Center for Immuno-Oncology at the National Cancer Institute (NCI); however, we often accrue upwards of 200 attendees from across the NCI keen on gaining valuable insights into the latest scientific discoveries and technological innovations helping to shape the future of cancer immunotherapy.
CIO Distinguished Lecture Series Speakers include:
2022: Drs. Lisa Butterfield, Robert Ferris, Marcella Maus, Greg Delgoffe, Sandra Demaria, Jeffrey Miller, and William Murphy
2023: Drs. Robert Herman Vonderheid, Michael Caligiuri, Christopher Klebanoff, Elisabeth de Vries, Tak Mak, Saman MalekiVreki, Jeffrey Miller, and Cassian Yee
2024: Drs. Bernard Fox, Thomas Marron, Lawrence Fong, Saul Priceman, Jennifer Guerriero, Catherine Wu, and Franco Marincola