Kylie J. Walters, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Bldg. 538/Room 167
- Frederick, MD 21702
- 301-228-4374
- kylie.walters@nih.gov
RESEARCH SUMMARY
Dr. Walters is an internationally renowned structural biologist who has made seminal discoveries of how targeted protein degradation occurs in cells. Her laboratory has applied a multidisciplinary approach that includes cutting-edge cellular and structural biology methods, including CRISPR-based gene editing, NMR spectroscopy, and cryoelectron microscopy, to uncover how the proteasome recognizes and processes its substates. Major discoveries include identifying Rpn1 and Rpn13 as proteasome substrate receptors as well as a binding site for E3 ubiquitin ligase E6AP/UBE3A at the proteasome in Rpn10. These findings have led to new therapeutic strategies for cancer which she is pursuing by using structure-based drug design.
Areas of Expertise

Kylie J. Walters, Ph.D.
Research
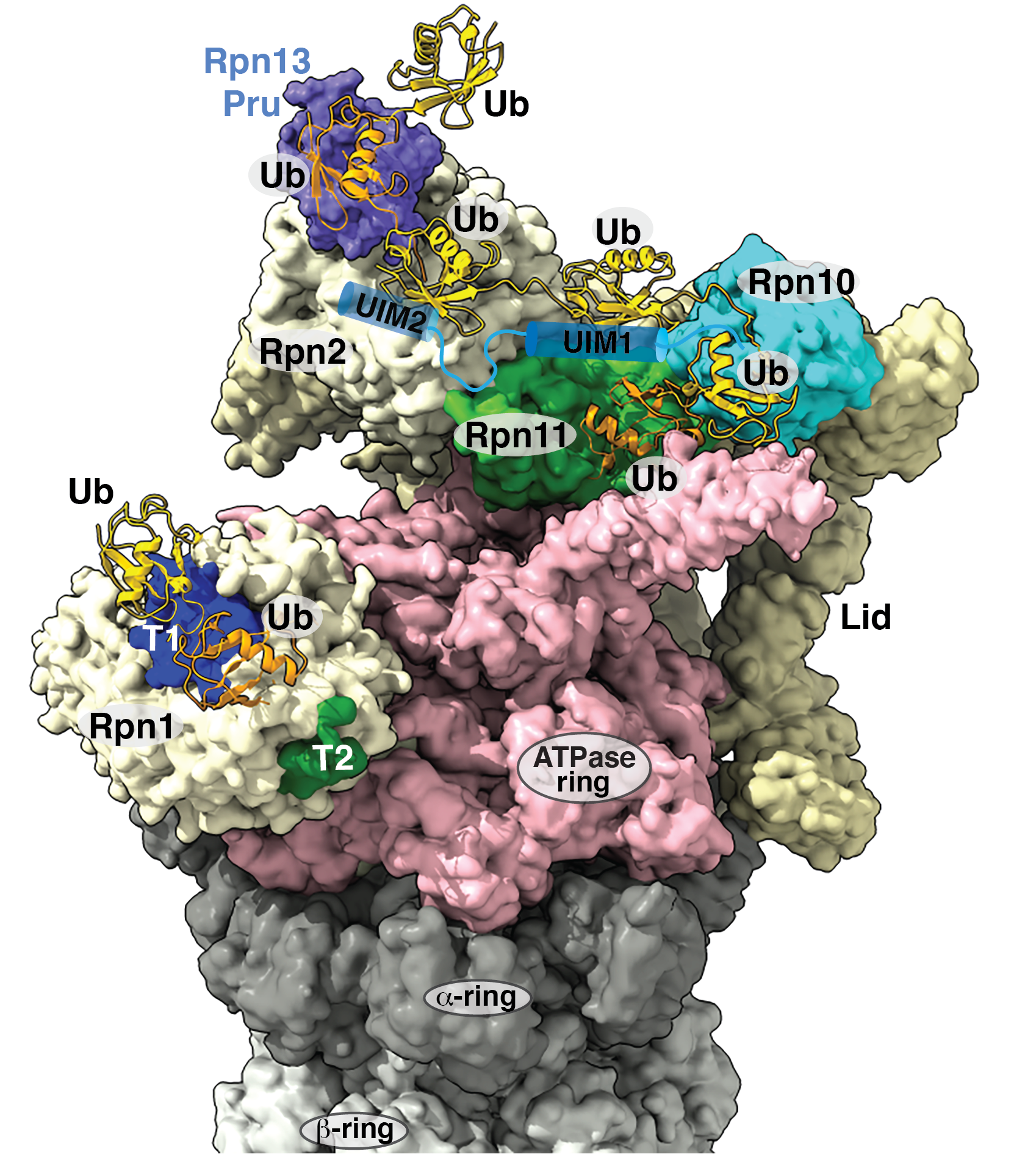
The Walters lab studies the structural and mechanistic basis of ubiquitin signaling events, proteasome function, and protein quality control. We use an interdisciplinary approach that merges functional cell-based assays with a variety of biophysical and structural biology techniques to define the mechanistic underpinnings of how protein lifetimes are regulated in cells. We have helped to identify the mechanisms of substrate recognition by the proteasome, identifying Rpn1 and Rpn13 as substrate receptors that bind to ubiquitin chains directly and to the shuttle factors that deliver ubiquitinated substrates to the proteasome. By using NMR spectroscopy, we have solved structures of these receptors complexed with ubiquitin chains and shuttle factors. We have also used cryoelectron microscopy to study ubiquitin chain binding to the 26S proteasome.
We discovered that Rpn13 is targeted in its ubiquitin-binding domain by cell permeable molecules that restrict cancer cell proliferation. We generated CRISPR cell lines that have revealed the importance of Rpn13 at the proteasome and its requirement for triggered apoptosis by these small molecules. Small molecules that inhibit the catalytic core particle of the proteasome are approved for treatment of hematological cancers and the targeting of Rpn13 is expected to function synergistically with these core particle inhibitors. We are further developing other Rpn13-targeting molecules by using structure-based drug design methods.
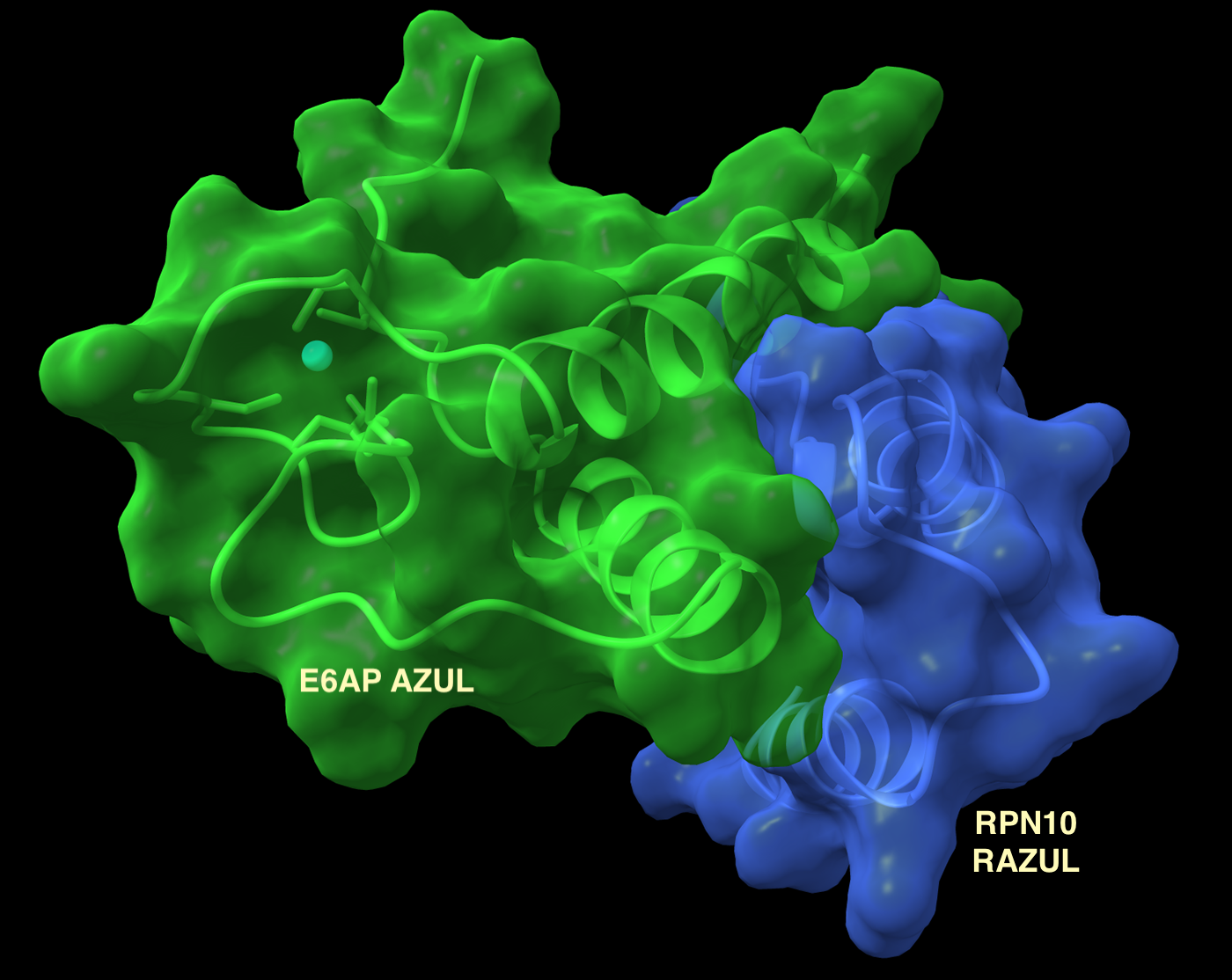
In a related project, we recently identified a binding site for the E3 ubiquitin ligase E6AP/UBE3A at the proteasome in substrate receptor Rpn10. This functional domain is the first found at the proteasome to recruit ubiquitination machinery. E6AP is targeted by the human papilloma virus E6 oncoprotein leading to degradation of tumor suppressor p53 and its dysfunction is also associated with prostate cancer, Angelman syndrome, and autism spectrum disorders. We continue to study the role of E6AP at the proteasome as well as other protein-protein interactions in the ubiquitin-proteasome pathway to understand the determinants of protein targeting for degradation as well as the mechanistic features that go awry in these pathways during carcinogenesis and neurological disorders.
Publications
- Bibliography Link
- View Dr. Walters' PubMed Summary.
hRpn13 shapes the proteome and transcriptome through epigenetic factors HDAC8, PADI4, and transcription factor NF-κB p50
High-throughput assay exploiting disorder-to-order conformational switches: application to the proteasomal Rpn10:E6AP complex
Rpn1 provides adjacent receptor sites for substrate binding and deubiquitination by the proteasome
Structure of E3 ligase E6AP with a novel proteasome-binding site provided by substrate receptor hRpn10
Biography

Kylie J. Walters, Ph.D.
Dr. Walters’ training in the field of biophysics began as an undergraduate at Wesleyan University, where she obtained HHMI funding to apply NMR methods to study the dynamic properties of DNA under the supervision of Dr. Irina Russu. She then obtained a Ph.D. in Biophysics from Harvard University, where with Dr. Gerhard Wagner, she developed and used methods to study protein structure, dynamics, and interaction mechanisms. As an American Cancer Society Postdoctoral Fellow with Dr. Peter Howley in the Pathology Department at Harvard Medical School, she began to apply structural biology methods to study the ubiquitin-proteasome pathway. In 2002, Dr. Walters joined the University of Minnesota as an Assistant Professor and was promoted to Associate Professor with tenure in 2008. During this time, Dr. Walters was selected for an American Cancer Society Research Scholar award for her work in protein quality control. In 2013, she moved to the Center for Cancer Research as a Senior Investigator, where she runs a vibrant research program that aims to apply basic science discoveries to translational benefit in the clinic. She has been awarded an NIH Director’s Award, NIH OD Honor Award, NCI Champions-Leading Diversity Award, and CCR top scientific advance. She currently serves on the CCR Science Board, Editorial Board of PLOS Biology, Advisory Board for the journal Structure, and has served as Guest Editor for PNAS and Current Opinions in Structural Biology.
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
Vasty Osei Amponsa and Kylie Walters were featured in a Molecular Cell Meet-the-Authors article.
Nov. 2020: Congratulations to Dr. Xiang Chen for his publication of how unanchored ubiquitin chains bind the proteasome – so-entering the world of cryoEM!
Congratulations to Dr. Anne Kaplan for getting a position at Aurobindo; we will miss you!
Congratulations to Dr. Olumide Kayode for getting a position with Revolve Biotechnologies; you will be missed!
August 2020: Congratulations to lead author Vasty Osei-Amponsa for making the cover of MCB for her studies of Rpn13 Pru domain and UCHL5 requirement in the clearance of ubiquitinated proteins and RA190 targeting.
May 5th 2020: Congratulations to Xiuxiu Lu and Danielle Ebelle on revealing the mechanism of how hRpn13 binds K48 ubiquitin chains, published today in Structure, with an excellent commentary by Jeroen Roelofs.
March 10th 2020: Congratulations to Gwen Buel and Xiang Chen on their discovery of a binding site for E6AP at the proteasome, published today in Nature Communications.
Members of the group celebrate achievements of 2019 at a restaurant in downtown Frederick.
Congratulations to Dr. Xiuxiu Lu for receiving a Best Poster Award at the 2018 NCI Structural Biology Retreat
Congratulations to Dr. Xiang Chen and Dr. Kylie Walters for receiving CCR Federal Technology Transfer Awards
Congratulations to Dr. Gwen Buel for being selected as a winner of NIH Fellows Award for Research Excellence (FARE) 2019 competition.
Congratulations to Dr. Xiang Chen for being selected as a winner of NIH Fellows Award for Research Excellence (FARE) 2017 competition. Xiang used this award to attend and present a poster at Cold Spring Harbor Laboratory Conference “The ubiquitin Family” on April 18-22, 2017.
Congratulations to Dr. Xiuxiu Lu for being selected to give an oral presentation at the ASBMB 2017 meeting in Chicago on April 22-26.
Dr. Vinidhra Sridharan presented a poster at the Cold Spring Harbor Laboratory Conference “The Ubiquitin Family”, April 18-22, 2017.