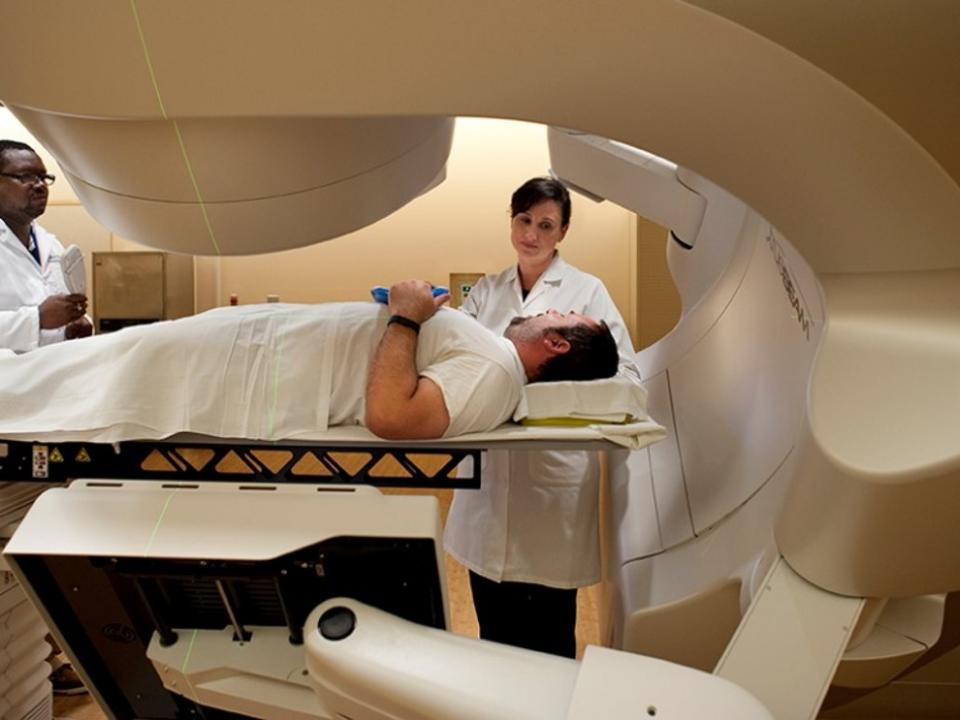
Although outcomes are generally excellent with modern radiation therapy for the treatment of localized prostate cancer, our team is working to develop and validate cutting-edge technologies to improve upon these outcomes. We conduct clinical trials treating men with localized prostate cancer and men with locally recurrent prostate cancer after surgery or prior radiation therapy.
Radiation Oncology Prostate Cancer Clinical Research Team
Our highly trained expert team is dedicated to conducting innovative clinical trials to improve curative treatment options for men with prostate cancer.
Radiation Oncology Prostate Cancer Clinical Research Team
About
Our clinical team develops novel applications of radiotherapy to treat prostate cancer with a specific focus on translational research, focal radiotherapy, local drug delivery, imaging guidance, and the management of recurrent prostate cancer. The trials implemented by our group prioritize improving cure rates while maximizing patients’ quality of life after treatment.
All of our trials take place at the NIH Clinical Center in Bethesda, MD, the world's largest research hospital, and through telehealth. Once you are enrolled in a clinical trial at the Clinical Center, medical care is free.
Have questions or need help finding a trial that is right for you?
Please contact Theresa Cooley-Zgela, RN, or Debbie Nathan, RN, via phone or email at 301-495-5457 or