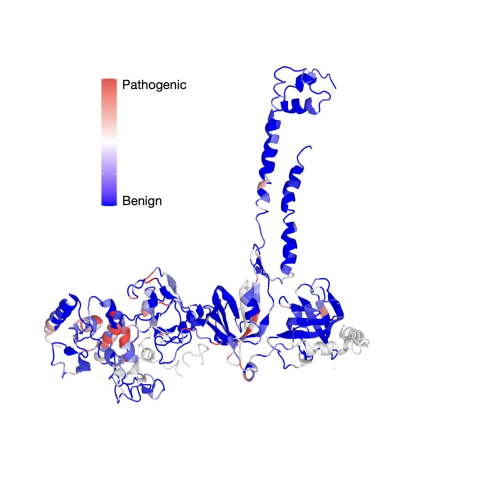
Image of the BRCA2 protein with a sliding scale of color indicating the regions containing certain variants. Blue regions contain benign variants, while red regions contain pathogenic regions.
A team of researchers led by Shyam K. Sharan, Ph.D., Senior Investigator in the Mouse Cancer Genetics Program, has categorized over 6,500 possible single nucleotide variants (SNVs) in the BRCA2 protein as either benign or potentially cancerous. The study was published in Nature on February 13, 2025.
BRCA2 is a protein that is involved in repairing DNA and maintaining the integrity of the genome in mammals, and variants in the gene that encodes BRCA2 are linked to hereditary breast and ovarian cancers, among others. Only a small number of SNVs have been classified to date.
The team, including first author and postdoctoral fellow Sounak Sahu, Ph.D., used CRISPR-Cas9, a technology that allows scientists to edit DNA in the genome, to integrate the human BRCA2 gene into a population of cells in the lab containing the mouse genome and engineer a single BRCA2 variant into each cell. The researchers then studied the effects of the variant on the cell’s activity and function and classified the variant using a seven-tier categorization system ranging from benign to pathogenic.
The findings of this study were meaningful for two primary reasons, Sharan said. The first is the number of variants that were identified. “Initially we were looking at tens, then hundreds of variants, but the number of new variants that have been identified in the human population is now in the thousands. In this study, we have examined more than 6,500 variants, which is a major increase from how many we could analyze using previous technology.”
Specifically, CRISPR-Cas9 technology can perform a process called saturation genome editing, which is a technique that allows researchers to assess the consequences of every possible variant in a genome by replacing each nucleotide, or basic building block of DNA, with the other three types of nucleotides to study the consequences.
The findings of this study and future research will also be impactful for patients. Knowing if a variant is pathogenic or benign will allow physicians to make effective decisions on whether intervention is needed or not.
“If someone inherits a mutation and our work shows that it is benign, you can imagine the relief it will give them,” Sharan said.
The researchers are now working on classifying other regions of the BRCA2 gene as well as gathering further information on variants with uncertain significance. Sharan’s lab also aims to transition from studies on molecular cellular processes to functional studies of the impact of variants on cancer risk in humans. They are working on forming consortiums to combine information and data from studies throughout the world, including from collaborators at institutions in Cyprus, France and Denmark, to give researchers more confidence in classifying the significance of BRCA2 variants.
Sharan concluded, “This is a very extensive project, and we are grateful to our collaborators for making it possible. It’s very exciting to see all this come together into something that could make a bit of difference in cancer screening in the future.”