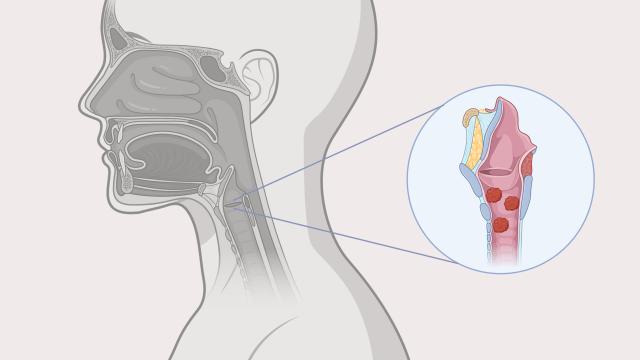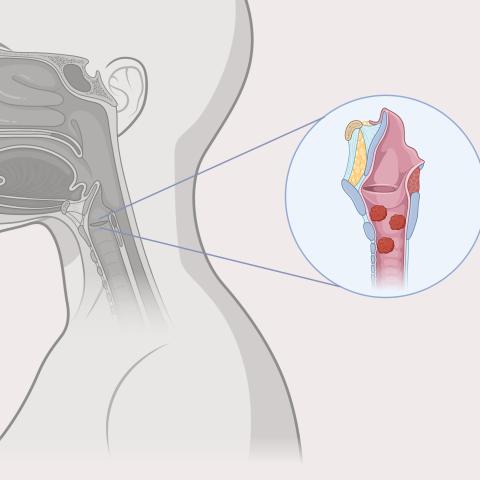
An illustration of papillomas in the throat, caused by recurrent respiratory papillomatosis.
Results from a phase 1/2 clinical trial led by CCR researchers show that treatment with PRGN-2012, an investigational gene therapy, resulted in a robust clinical benefit with significant reductions in the need for surgeries for adult patients with recurrent respiratory papillomatosis (RRP). The findings from the clinical trial were published January 17, 2025, in The Lancet Respiratory Medicine. The phase 1/2 study provided the pivotal registrational data for submission of a Biologics License Application to the U.S. Food and Drug Administration (FDA). If approved, PRGN-2012 has the potential to be the first FDA-approved treatment for RRP.
RRP is a rare disorder that is caused by infection with human papillomavirus (HPV) type 6 or 11. It causes growth of papillomas, or non-cancerous tumors, throughout the respiratory and digestive tracts.
“The condition can be debilitating for several reasons,” explained Scott Norberg, D.O., Associate Research Physician in the Center for Immuno-Oncology, first author of the study and principal investigator of the clinical trials. It affects the voice and breathing of people who have it, causing higher levels of social anxiety and decreased social functioning. People must often turn to surgical intervention to control the papillomas, which is time-consuming and can be expensive; repeated surgeries can also damage vocal cords and airways over time. Many of the participants in the study had received hundreds of procedures over their lifetimes to control RRP.
PRGN-2012 is an innovative gene therapy based on Precigen’s gorilla adenovector technology designed to modulate the immune system in vivo. PRGN-2012 is engineered to induce HPV 6 and 11 specific immune responses in RRP patients to help control the tumors.
The clinical trial, led by Norberg and Clint T. Allen, M.D., Senior Investigator in the Surgical Oncology Program and senior author on the publication, was a combined phase 1/2 study evaluating the safety and efficacy of PRGN-2012. The study enrolled 38 adult participants with RRP who had required three or more interventions in the previous year. The participants received four subcutaneous injections of PRGN-2012 over a 12-week interval.
The researchers found that 18, or just over half of the 35 participants that received the recommended phase 2 dose, experienced a protocol-defined complete response, meaning they did not need an intervention to control their RRP for 12 months after treatment. Based on the team's last data analysis, 15 of these participants required no further interventions after a median follow-up of 22 months. Finally, more than 85% of these patients experienced a decrease in necessary interventions after receiving the treatment regimen compared to their prior treatment history.
Success of the phase 1 portion of the trial had prompted breakthrough therapy designation from the FDA. The two trials’ findings represent a positive development for RRP patients.
“We feel this really changes the way that adults with RRP in the United States will be treated, and we think this treatment has the possibility of eliminating many procedures that patients would have to receive otherwise,” Norberg said.