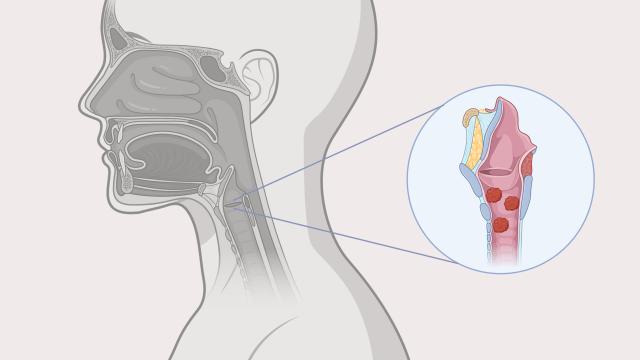
An illustration of papillomas in the throat, caused by recurrent respiratory papillomatosis (RRP). Newly approved Papzimeos is the first non-surgical treatment for RRP. Credit: Image created by Linda Wang with BioRender.
The United States Food and Drug Administration (FDA) has granted full market approval for Papzimeos (zopapogene imadenovec-drba), a groundbreaking non-replicating adenoviral vector-based immunotherapy designed for adult patients with recurrent respiratory papillomatosis (RRP). This treatment is the first of its kind, and it marks a significant advancement in the treatment of this rare and debilitating disease.
The study was led in CCR by lead investigators, Scott M. Norberg, D.O., and Clint T. Allen, M.D., under a collaborative research and development agreement (CRADA) with the biopharmaceutical company Precigen. Pivotal data was published in The Lancet Respiratory Medicine.
The approval was completed under Priority Review and the product received both Orphan Drug designation and Breakthrough Therapy designation.
RRP is a rare, debilitating, and potentially life-threatening disease of the upper and lower respiratory tract caused by chronic HPV 6 or HPV 11 infection. RRP can lead to severe voice disturbance, a compromised airway, and recurrent post-obstructive pneumonias. Until now, management of RRP primarily consisted of repeated surgeries, which do not address the root cause of the disease and can be associated with significant morbidity as well as significant patient and health system burden.