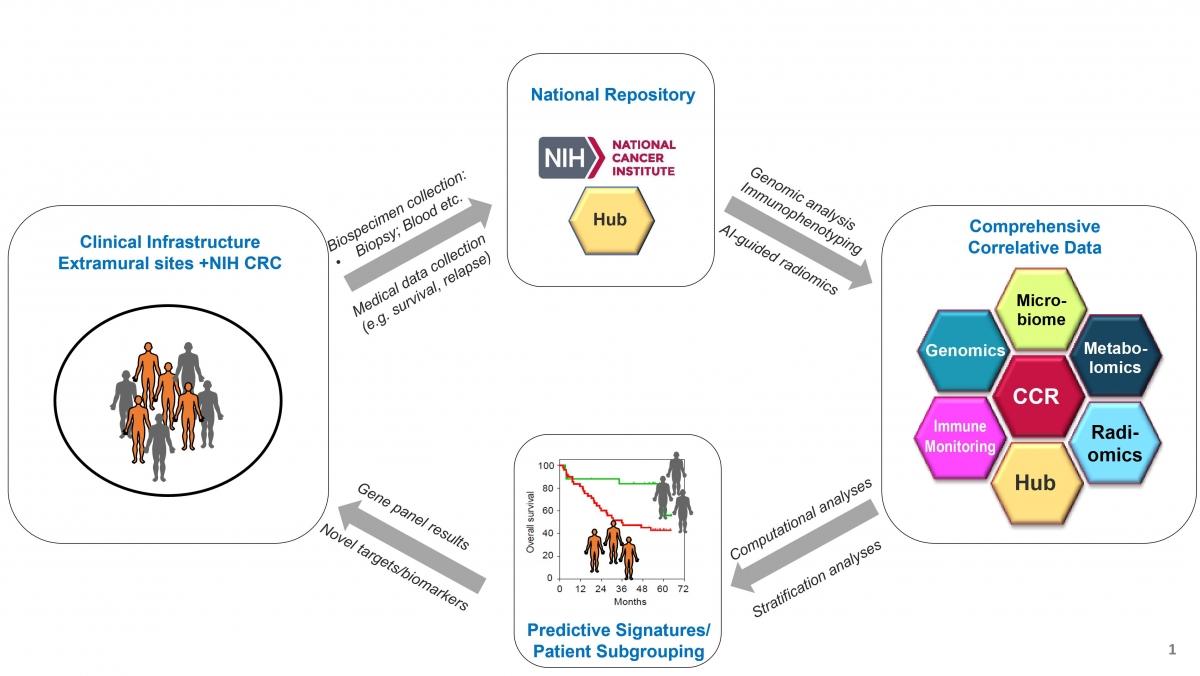
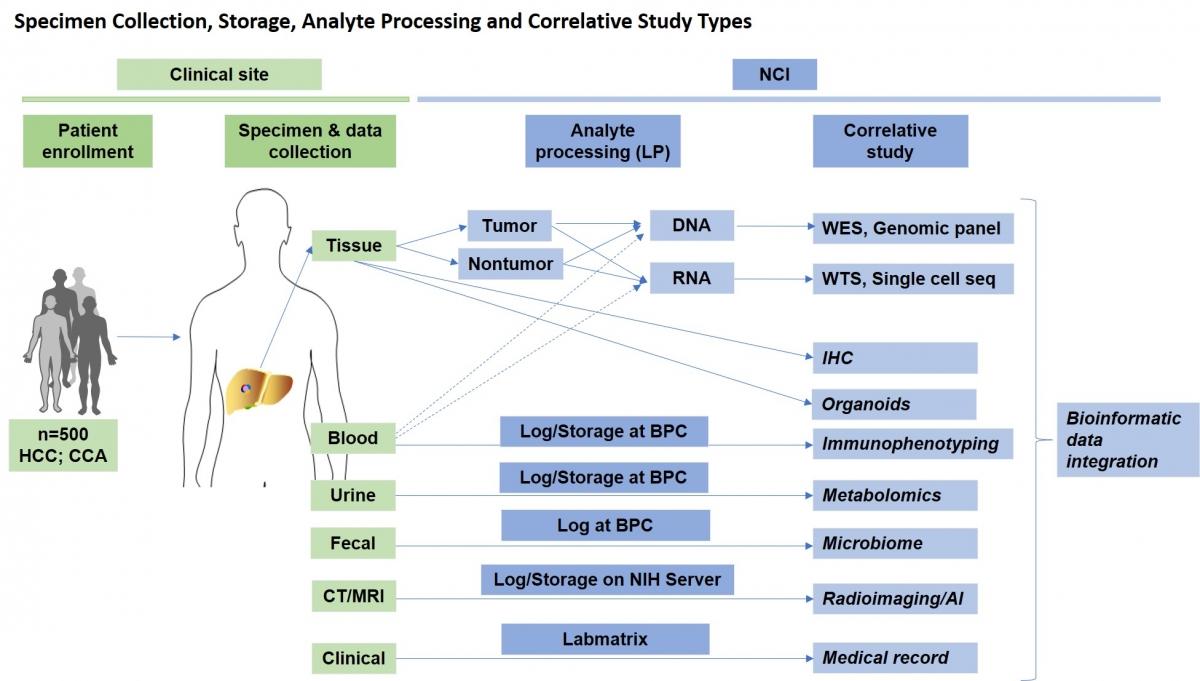
Overall Goal: To build an NCI Intramural Research Program (IRP)-based collaborative translational science network of liver cancer clinical trial data, accompanying biospecimens and correlative laboratory data.
Specific Aim: Determine why immunotherapy is effective in certain patients but not in others and to use this information to develop novel therapies.
Background: Primary liver cancer (PLC) is the 2nd most common cause of cancer-related death worldwide and the one cancers with the fastest rising incidence and mortality in the U.S. PLC consists of two main histological subtypes, i.e., hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA). PLC is clinically and biologically heterogeneous which has impeded biological assessment and clinical treatment. Despite considerable efforts towards improving diagnosis and development of new treatment modalities, the improvement of PLC patient survival is minimal. PLC therefore remains among the most difficult-to-treat malignancies, with a 5-year survival rate of less than 15% in the United States. Thus, it is imperative that new treatment modalities are developed to limit cancer development and treat advanced PLC.
Study Focus: Immunotherapy is a promising new approach in PLC treatment. At the moment, it is difficult to determine which patient may benefit from immunotherapy, due in large part to the lack of large comprehensive studies, biobank resources of specimens and biospecimen collection in clinical trial protocols, which deter our ability to understand and define critical genomic or genetic factors that contribute to patient response.
Study Objectives: We will collect PLC patient specimens and clinical data from those undergoing immunotherapies at NIH Clinical Center and clinical sites across the U.S. to develop predictors for (a) response or resistance to immunotherapy and (b) acquired resistance to immunotherapy.
- Acronym: NCI CLARITY: Cancers of the Liver: Accelerating Research of Immunotherapy by a TransdisciplinarY Network (Note: The study aims to provide ‘clarity’ on who responds/does not respond to immunotherapy.)
- Protocol#: 20-C-0006 (on NIH clinical trials.gov); patients should be enrolled on an immunotherapy trial at NIH CRC or at a participating site and will dually enroll on this natural history (observational) study; 5-year study; estimated enrollment: 500 individuals.
- Includes the two main forms of primary human liver cancer: HCC and CCA.
- Large multi-institutional and multidisciplinary study involving collaboration between NCI and several domestic clinical institutions.
- A biorepository of multiple human biospecimens (tissue biopsy, blood, urine and stool) longitudinally collected (baseline, on treatment, post treatment and follow-up) for multiple downstream correlative studies including large-scale genomics.
- Identify patient subgroups, biomarkers and/or molecular signatures associated with immunotherapy response.
Primary liver cancer IO patients will be enrolled at clinical sites where biospecimens and medical data will be collected and sent to NCI. At NCI, biospecimens will be processed for respective correlative studies performed by NCI Investigators and will be integrated with bioinformatic approaches.
The clinical portion of the study is on the left (green) while the research portion is on the right (blue). Please note that BPC stands for Biospecimen Processing Core.
Tumor biology and immune infiltration define primary liver cancer subsets linked to overall survival after immunotherapy.
Anuradha Budhu, Erica C. Pehrsson, Aiwu He, Lipika Goyal, Robin Kate Kelley, Hien Dang, Changqing Xie, Cecilia Monge, Mayank Tandon, Lichun Ma, Mahler Revsine, Laura Kuhlman, Karen Zhang, Islam Baiev, Ryan Lamm, Keyur Patel, David E. Kleiner, Stephen M. Hewitt, Bao Tran, Jyoti Shetty, Xiaolin Wu, Yongmei Zhao, Tsai-Wei Shen, Sulbha Choudhari, Yuliya Kriga, Kris Ylaya, Andrew C. Warner, Elijah F. Edmondson, Marshonna Forgues, Tim F. Greten, and Xin Wei Wang. Cell Reports Medicine, May 23, 2023.
Intramural Leadership Team
Tim Greten, M.D.
Xin Wang, Ph.D.
Anuradha Budhu, Ph.D.
Intramural Investigators
Frank Gonzalez, Ph.D.
David Kleiner, M.D., Ph.D.
Natalie Porat-Shliom, Ph.D.
Eytan Ruppin, M.D., Ph.D.
Giorgio Trinchieri, M.D.
Brad Wood, M.D.
Intramural Research Staff
Aryan Neupane, Ph.D. – Bioinformatician
TBD – Data Manager
TBD – Technician
Intramural Clinical Staff
Stephanie Hicks – Research Nurse
Melissa Walker – Research Nurse
Angelicia Garrison – Patient Care Coordinator
Grace-Ann Fasaye – Genetic Counselor
Intramural Affiliate Staff
Jason Levine, M.D. – IT and Clinical Informatics
Ming Li – IT Developer
W. Douglas Figg Sr., Pharm.D. – Biospecimen Processing Core
Paula Carter, BSN, RN – Biospecimen Processing Core
Trung Pham, MS – Biospecimen Processing Core
Extramural Team
R. Katie (Robin) Kelley, M.D., University of California at San Francisco
Lipika Goyal, M.D., Massachusetts General Hospital
Aiwu (Ruth) He, M.D., Georgetown University
Hien Dang, Ph.D., Thomas Jefferson University
Yujin Hoshida, M.D., Ph.D., University of Texas at Southwestern and Parklands Hospital
Adam Burgoyne, M.D., Ph.D., University of California San Diego
Sukeshi Patel Arora, M.D., Mays Cancer Center, UT Health San Antonio
Kevin Kim, M.D., University of Maryland at Baltimore
Extramural Pathology Lead
Ryan Gill, M.D., Ph.D., University of California at San Francisco
Vikram Deshpande, MBBS, Massachusetts General Hospital
Bhaskar Kallukury, M.D., Georgetown University
Extramural Radiology Lead
Spencer Behr, M.D., University of California at San Francisco
Reena C. Jha, M.D., Georgetown University