Chunzhang Yang, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 37, Room 1142E
- Bethesda, MD 20892
- 240-760-7083
- 240-541-4466
- chungzhang.yang@nih.gov
RESEARCH SUMMARY
Dr. Chunzhang Yang studies the genetic and molecular basis of brain tumors, and leads the Molecular and Cell Biology Research Program at the Neuro-Oncology Branch (NOB). The goal of his research is to understand the vulnerabilities of brain tumors and identify biological signatures—which can be used to generate experimental therapeutics that improve the current standard of care for patients.
His lab utilizes cutting-edge molecular biology techniques and state-of-the-art preclinical models to understand the essential molecular pathways in brain tumors. He also conducts mechanistic studies on these key pathways, which could be inhibited in brain tumors to elicit more cell death, improve disease outcomes, and diminish therapeutic resistance. The primary approaches that Dr. Yang’s team uses include functional analysis of cancer biology, identification of actionable therapeutic targets, and development of experimental therapeutics for next step clinical application.
Explore the NOB's Research Programs >
Areas of Expertise

Chunzhang Yang, Ph.D.
Research
Within the scope of his glioma research at the Neuro-Oncology Branch (NOB), Dr. Yang has designed a multifaceted approach to not only understand disease biology, but also steer laboratory research in a translationally-relevant direction.
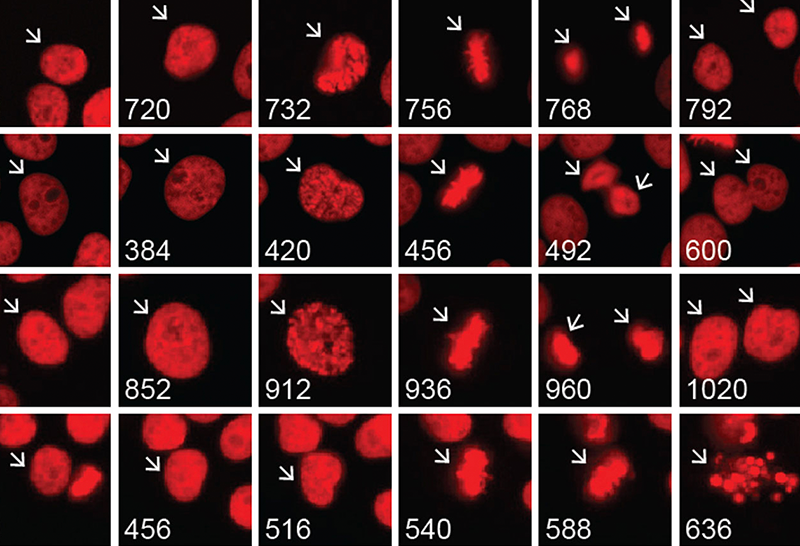
The first primary focus within Dr. Yang’s lab involves using DNA repair inhibitors to sensitize glioma cells to radiation and chemotherapy. The goal of this work is to use multi-omic techniques with patient derived cell lines, tissue specimens, and bioinformatics to shed light on the transcriptomic, metabolic, and biochemical differences between glioma subtypes.
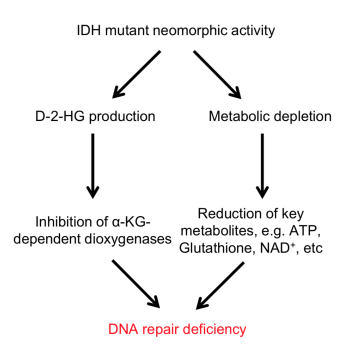
Additionally, Dr. Yang has been focused on the neomorphic activity (that is, the change in function) of the IDH enzyme, as well as its oncometabolite 2-hydroxyglutarate (2-HG) and its role in cancer biology and therapeutic vulnerabilities. Dr. Yang’s recent findings suggest that administering an FDA-approved PARP inhibitor (a DNA repair inhibitor) could result in synthetic lethality in IDH-mutant glioma cells, which improves therapeutic response with reduced tumor expansion.
Another broad research endeavor in Dr. Yang’s laboratory is identifying the metabolic and biological vulnerabilities of IDH-mutant glioma cells, in order to understand how these cells develop a protective shield and avoid being maximally impacted by therapy. Research has shown that NRF2, a transcription factor, could be a critical driver for survival in IDH-mutant tumors, and Dr. Yang’s findings suggest that targeting NRF2 results in more profound cytotoxicity in preclinical mouse models. Dr. Yang is also collaborating with other research groups within the NCI Center for Cancer Research’s Neuro-Oncology Branch (NOB) and the NCI more broadly to develop reliable IDH-mutant glioma mouse models that will be essential for studying the disease and potential treatments in a preclinical setting.
Publications
- Bibliography Link
- View Dr. Yang's Google Scholar Profile
Biography

Chunzhang Yang, Ph.D.
Dr. Yang obtained his Ph.D. in neurobiology from Peking University in China immediately after earning a Bachelor of Science in medicine at the same institution. Motivated by a family member’s brain tumor diagnosis, he moved to the NIH shortly after completing his degrees to pursue a postdoctoral fellowship at the Surgical Neurology Branch under National Institutes of Neurological Disorders and Stroke. During this time, Dr. Yang led several research projects to understand the biology of neurological diseases and central nervous system tumors, in hopes of developing a therapeutic regimen optimized for these diseases. Following his postdoctoral fellowship in 2016, he joined the Neuro-Oncology Branch (NOB) as an investigator, and has led the Molecular and Cell Biology Program ever since.
In addition to his research, Dr. Yang is also part of several societies, such as the American Association for Cancer Research and the Society for Neuro-Oncology. He has received several awards during his time at the NIH, including a Distinguished Scientist Award and a Young Investigator Award for his postdoctoral research.
Select Societies and Initiatives
- NIH DNA Repair Interest Group
- American Association for Cancer Research (AACR)
- Society for Neuro-Oncology (SNO)
- Society of Chinese Bioscientists in America (SCBA)
Job Vacancies
| Position | Degree Required | Contact Name | Contact Email |
|---|---|---|---|
| Postdoctoral Fellow - cancer biology, functional genomics | Ph.D. or equivalent | Chunzhang Yang | yangc2@nih.gov |
Team
News
New Drug Enhances Chemotherapy to Control Glioblastoma Cell Growth and Target Treatment Resistance
February 12, 2024
This drug disrupts cell cycle timing in brain tumor cells and makes them more vulnerable to chemotherapy, which improves survival in preclinical models. Read more >
New Study Decodes the Genetics of Brain Tumors to Develop More Precise Therapies
February 27, 2023
Cancer researchers have identified an effective treatment in mouse models for targeting gliomas carrying genetic abnormalities known as IDH mutations. Read more >
Exploiting Brain Cancer Cells’ Vulnerabilities to Combat Treatment Resistance
As head of the Molecular and Cell Biology Program, Dr. Chunzhang Yang investigates whether genetic mutations in cancer cells could serve as therapeutic targets. Read more >
Celebrating National Mentoring Month
January 31, 2020
We celebrate our impactful mentors in the Neuro-Oncology Branch for National Mentoring Month. Our postdoctoral fellows share their love for science and how their mentors have made their experience at NIH more rewarding. Read more >
PARP Inhibitors May be Effective in Brain, Other Cancers with IDH Mutations
April 24, 2017
Two separate studies revealed insights into the function of genetic mutations commonly found in a form of brain cancer—and, in the process, identified a potential new treatment strategy. Read more >
Lab Life
Dr. Yang Liu won a poster award in the 2022 27th annual meeting of the Society for Neuro-Oncology.
Our publication won "The Best of the AACR Journals" because it was the most-cited article in Cancer Research. This image was taken during the 2019 American Association for Cancer Research Annual Meeting.
Postdoc Dr. Yang Liu won the best poster award during the 2018 Annual Symposium for the Society for Neuro-Oncology.
On May 22, 2018, postdoc Dr. Yanxin Lu gave birth to a baby boy Ziyuan Yue (Jefferson).
Postdoc Dr. Yanxin Lu gave an oral presentation about her laboratory work in the Neuro-Oncology Branch during the 2017 American Association for Cancer Research Annual Meeting.