This drug disrupts cell cycle timing in brain tumor cells and makes them more vulnerable to chemotherapy, which improves survival in preclinical models.
Raleigh McElvery, Scientific Communications Editor
February 12, 2024
Cancer cells have a unique ability to replicate themselves much faster than healthy cells. They speed up their cell cycle so they can grow quickly.
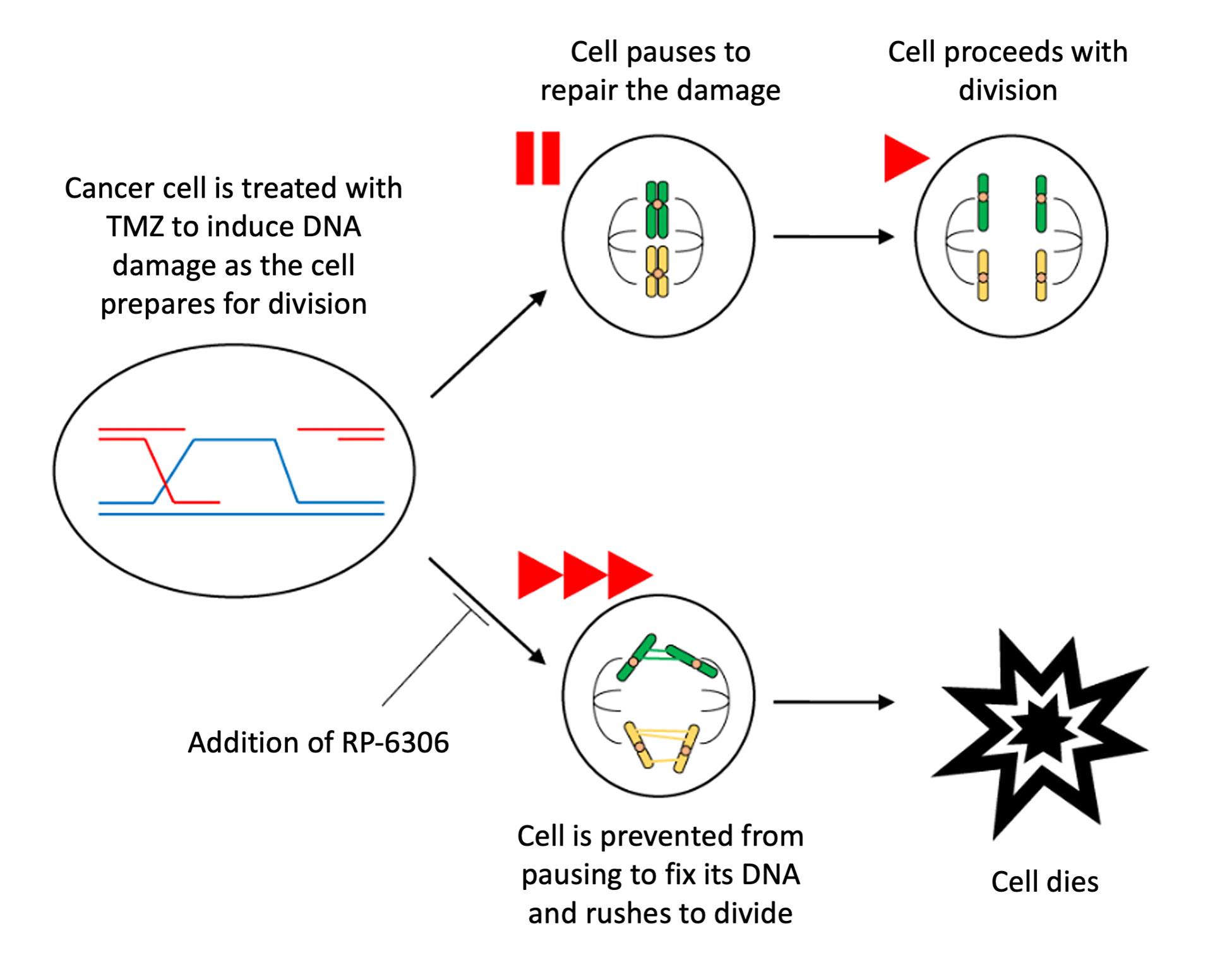
Many chemotherapies work by damaging cancer cells' DNA. This damage accumulates as the cells divide, affecting their function and eventually killing them. The chemotherapy temozolomide (TMZ) is the standard treatment for glioblastoma, the most common primary brain cancer in adults. However, this therapy often leads to only modest improvements in disease outcome. That’s because glioblastomas, and other cancers, have learned to pause their cell cycle once they sense DNA damage. This gives them a chance to perform repairs before replicating themselves—preventing them from passing potentially lethal damage on to their daughter cells.
To make glioblastomas more sensitive to TMZ and other treatments, researchers in the NCI Center for Cancer Research’s Neuro-Oncology Branch (NOB) learned to manipulate the timing of the cell cycle. In a new study published in Neuro-Oncology, a team from the NOB’s Molecular and Cell Biology Research Program used a drug called RP-6306 (Lunresertib) to rush glioblastoma cells through cell division without giving them a chance to fix the DNA damage inflicted by TMZ—ultimately killing them. Combining TMZ and RP-6306 proved more effective than TMZ treatment alone in mouse models of treatment-resistant glioblastoma.
“Our study presents a new research angle that will hopefully help improve outcomes for glioblastoma patients,” says Chunzhang Yang, Ph.D., head of the Molecular and Cell Biology Research Program and the study’s senior author. “Altering cell cycle timing is a powerful approach that has the potential to help people with many different cancers. Our study provides a great starting point.”
The researchers began by using a gene editing technique called CRISPR to screen cells’ genomes and determine which genes were vital to TMZ resistance. One by one, they knocked out each gene to see which helped the cells survive TMZ exposure. They pinpointed a gene called PKMYT1 that protected cells from TMZ. When they depleted this gene, the cells became much more sensitive to chemotherapy.
“We are very proud of this technique, called a chemogenomic screen,” explains first author and NOB Visiting Fellow Fengchao Lang, Ph.D. “It’s an unbiased approach, meaning it’s an impartial method of scanning the genome for therapeutic targets. We were excited to identify the PKMYT1 gene as one of these possible targets.”
PKMYT1 is highly expressed in glioblastoma cells and often associated with worse prognosis in patients. This gene encodes an enzyme called Myt1, which helps pause cell division if there is DNA damage that needs to be repaired. According to Drs. Yang and Lang, Myt1 functions like a brake on a car.
“If the cell senses an issue, it can hit the brake and fix the problem,” Dr. Yang explains. “But if we remove the brake—in this case, Myt1—then the car continues to run until it breaks down.”
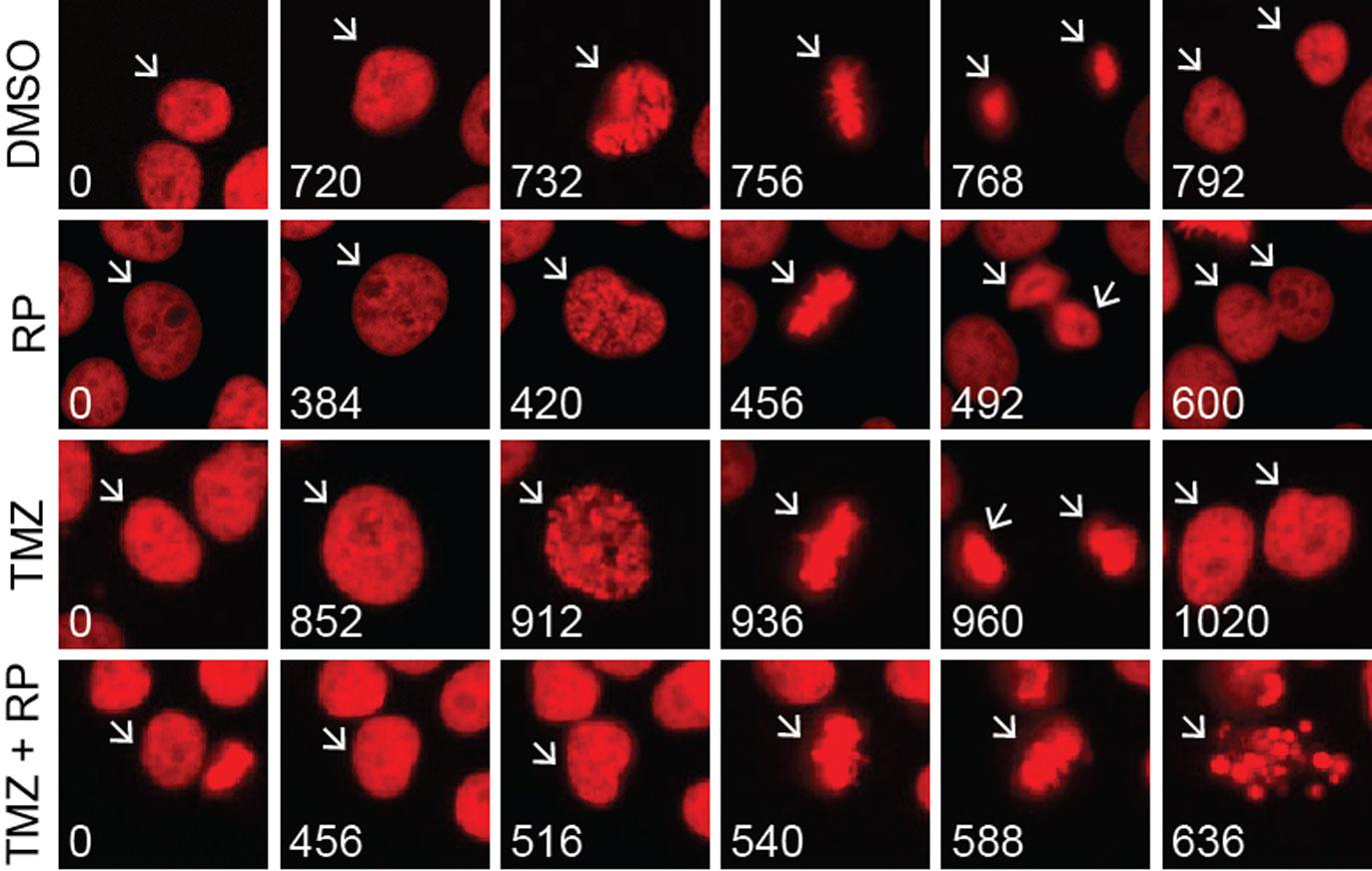
Drs. Yang and Lang used several methods to validate this hypothesis and render glioblastoma cells more sensitive to TMZ. First, they employed a genetic approach called RNA silencing to decrease the amount of Myt1 in glioblastoma cells. Without Myt1, the cells could not endure TMZ exposure and eventually died.
Next, the researchers treated glioblastoma cells with both TMZ and RP-6306—a drug that prevents Myt1 from pausing cell division and addressing the DNA damage caused by TMZ. As the DNA damage accrued, the cancer cells were no longer able to survive.
To lay a foundation for future clinical trials, the researchers also performed tests in a mouse model of glioblastoma. Their TMZ and RP-6306 combination suppressed tumor growth and extended overall survival in the mice.
In addition to combining RP-6306 and TMZ, Drs. Yang and Lang also tested RP-6306 with other chemotherapies, including BCNU, busulfan, and dacarbazine. In all cases, adding RP-6306 was more effective at killing glioblastoma cells than chemotherapy alone.
Because RP-6306 sensitizes tumors to TMZ, doctors may prescribe patients lower doses of TMZ—which would decrease the likelihood of adverse side effects. The researchers also posit that RP-6306 would complement other therapies besides chemotherapy that work by damaging cancer cells’ DNA, such as radiation.
There are currently several clinical trials combining RP-6306 with other therapies to treat ovarian cancer, uterine cancer, and other advanced solid tumors. However, much less is known about its potential for treating brain tumors. The next step, Dr. Yang says, will be to investigate whether glioblastoma cells treated with RP-6306 will resort to another means of survival that doesn’t rely as much on Myt1. If so, the researchers will need to tweak their approach accordingly.
“We have to acknowledge that glioblastoma is a very tricky disease,” Dr. Yang says. “At this point, we know that RP-6306 will be effective in some TMZ-resistant glioblastomas. We hope our future research will provide additional evidence that RP-6306 is a good candidate to proceed to clinical study.”
Hear Dr. Yang Share His Work