Li Yang, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 37, Room 3134C
- Bethesda,, MD 20892-4255
- 240-760-6809
- yangl3@mail.nih.gov
RESEARCH SUMMARY
Dr. Yang’s research program focuses on the mechanisms underlying tumor-host interplay in cancer metastatic progression. Dr. Yang has contributed to the identification of the immune and inflammatory mediators in the anti- and pro-tumor functional switch of TGF-β, the epigenetic regulation of TGF-β signaling, mechanisms of myeloid cell recruitment in the tumor microenvironment, as well as myeloid TGF-β signaling in cancer immune surveillance and in inflammatory stroke.
As the head of the Tumor Microenvironment and Metastasis Section, Dr. Yang is particularly interested in how tumor suppressors modulate the host immune environment, and the epigenetic mechanisms responsible for tumor cell plasticity and metastatic colonization.
Areas of Expertise

Li Yang, Ph.D.
Research
Metastatic spread accounts for most cancer-associated deaths in patients, but treatment options are limited. How cancer cells acquire the competence to colonize distant organs is poorly understood. The goal of my research team is to identify the cause-effect mediators that link the genomic/epigenetic alterations and tumor microenvironment and options for therapeutic intervention. We collaborate with basic research laboratories as well as clinicians and epidemiologists.
We have several focused research areas:
Roles of tumor suppressors (TSs) in metastatic progression: TSs are powerful transcriptional and signaling regulators that negatively modulate cell proliferation and survival. As such, TSs counteract the growth promoting activity of oncogenes mainly through cell autonomous mechanisms. It is not clear whether TSs play a direct role in metastatic colonization. Our studies demonstrate that tumor suppressors are often silenced during metastatic progression through epigenetic mechanisms (e.g., promoter hypermethylation and histone modification). TS deregulation promotes the acquisition of plasticity and the ability of tumor cells to adapt to hostile tissue microenvironments, thus facilitating metastatic spread. We use integrated genome-wide genetic and epigenetic approaches to investigate the role of TSs during metastatic progression and the underlying mechanisms involving host inflammatory and immune responses.
Epithelial and myeloid TGF-β in cancer metastasis: TGF-β is a powerful metastasis promoter in later stages of cancer progression; however, it mediates growth inhibition in early stages. The factors mediating the functional change of TGF-β are largely unknown, which poses significant challenges to our understanding of TGF-β cancer biology and to the successful application of TGF-β-targeted therapy. We use focused genetic models in which TGF-β signaling is inactivated in specific cell types in the tumor and microenvironment, including host myeloid cells or stromal fibroblasts, to discover molecular mediators and pathways important in tumor-stroma crosstalk.
Immune microenvironment regulation of tumor dormancy: the ability of residual tumor cells to persist in a dormant state can occur during metastatic progression and/or following extended periods of clinical remission that may last decades. The mechanisms for tumor dormancy induction or tumor cell reactivation remain unclear. We are currently using in vitro and in vivo molecular imaging, single-cell RNA sequencing, and mouse models of breast cancer to investigate the molecular and cellular mechanisms mediated by the immune microenvironment in tumor dormancy.
Please contact Dr. Li Yang for information regarding the availability of postdoctoral and graduate fellowship positions in the lab. Graduate students may apply through the Graduate Partnership Program that sponsors doctoral students at NIH through partnerships with various universities, including the University of Maryland and Johns Hopkins University, and others as well.
Publications
- Bibliography Link
- View Dr. Yang's Complete Bibliography at NCBI.
Induction of DNMT3B by PGE2 and IL6 at Distant Metastatic Sites Promotes Epigenetic Modification and Breast Cancer Colonization
miR-130a and miR-145 reprogram Gr-1+CD11b+ myeloid cells and inhibit tumor metastasis through improved host immunity
TGF-β signaling in myeloid cells is required for tumor metastasis
Biography

Li Yang, Ph.D.
Dr. Li Yang is a Senior Investigator at the National Cancer Institute. She received her Ph.D. in the Department of Cancer Biology at Vanderbilt University, under the mentorship of Dr. David Carbone. Her dissertation research focused on COX-2 pathway in tumor progression, immune suppression, and the contribution of host myeloid cells to tumor blood vessel formation. She investigated TGF-β regulation of inflammation and tumor microenvironment during her postdoc research with Dr. Harold Moses. She joined NCI in 2009 and was tenured in 2016. Her research program is devoted to understanding the molecular mechanisms underlying tumor-stroma interaction during metastatic process.
Dr. Yang is a recipient of the Federal Technology Transfer Award, co-recipient of FLEX Program Awards for Principal Investigators, CCR, NCI, as well as U.S.-China Biomedical Collaborative Research Grant award.
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
Justin Gray was featured in an NIH SciBites video:
"How Dying Cancer Cells Fuel Tumor Growth"
Justin Gray, a graduate student in the NIH-Johns Hopkins GPP, awarded his Ph.D.
Justin completed his doctoral research in the Yang lab (July 2017 - June 2022) and is now working at BioNTech.
Covers
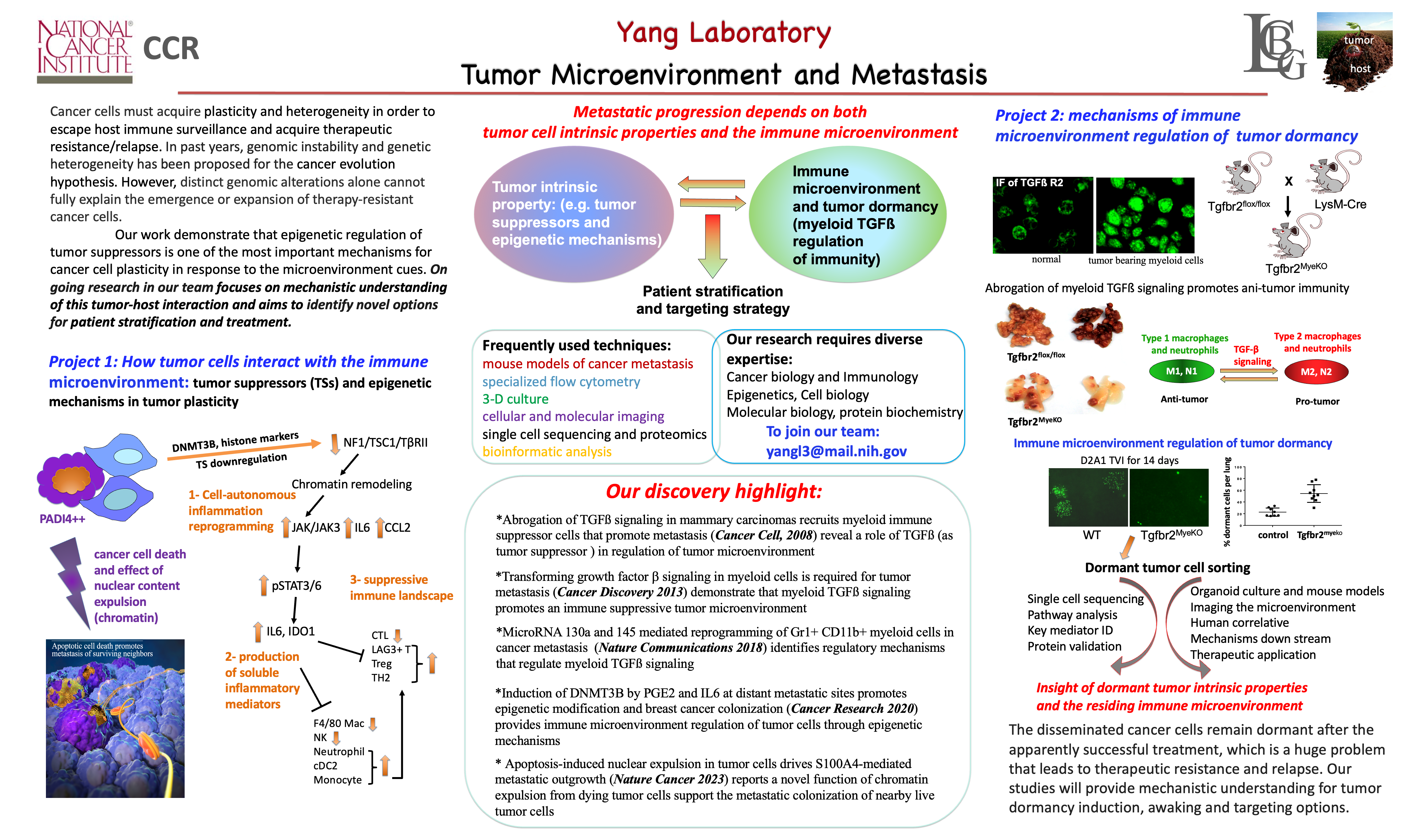
Apoptosis-induced nuclear expulsion in tumor cells drives S100A4-mediated metastatic outgrowth
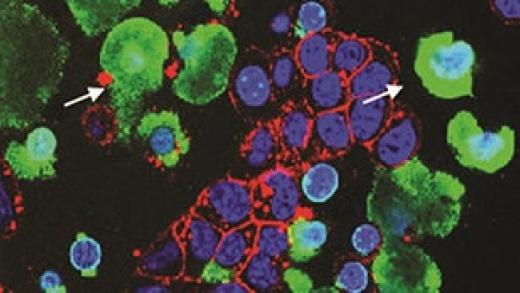
Most tumor cells undergo apoptosis in circulation and at the metastatic organ sites due to host immune surveillance and a hostile microenvironment. It remains to be elucidated whether dying tumor cells have a direct effect on live tumor cells during the metastatic process and what the underlying mechanisms are. Here we report that apoptotic cancer cells enhance the metastatic outgrowth of surviving cells through Padi4-mediated nuclear expulsion. Tumor cell nuclear expulsion results in an extracellular DNA–protein complex that is enriched with receptor for advanced glycation endproducts (RAGE) ligands. The chromatin-bound RAGE ligand S100a4 activates RAGE receptors in neighboring surviving tumor cells, leading to Erk activation. In addition, we identified nuclear expulsion products in human patients with breast, bladder and lung cancer and a nuclear expulsion signature correlated with poor prognosis. Collectively, our study demonstrates how apoptotic cell death can enhance the metastatic outgrowth of neighboring live tumor cells.
This article was also highlighted in the Editorial section of Nature Cancer.

DNA Methyltransferase 3B–Mediated Intratumoral Heterogeneity and Therapeutic Targeting in Breast Cancer Recurrence and Metastasis
The mechanisms of how cancer cells are selected and evolve to establish distant metastatic colonies remain unclear. Tumor heterogeneity and lack of biomarkers are some of the most difficult challenges in cancer biology and treatment. Here using mouse models for triple-negative breast cancer (TNBC) metastasis, we report heterogeneous expression of DNA methyltransferase 3B (DNMT3B) in both mouse and human primary tumors. High levels of DNMT3B were correlated with poor clinical outcomes in multiple human breast cancer datasets. Mechanistically, clonal cells with high DNMT3B (DNMT3BH) showed higher vimentin (VIM) expression and displayed enhanced epithelial-to-mesenchymal transition capacity. Deletion of VIM diminished the metastatic phenotype of DNMT3BH cells. Importantly, in preclinical mouse models in which the primary tumors were surgically removed, perioperative targeting of DNMT3B in combination with chemotherapy markedly suppressed tumor recurrence and metastasis. Our studies identify DNMT3B-mediated transcription regulation as an important mediator of tumor heterogeneity and show that DNMT3B is critical for tumor invasion and metastasis, reinforcing its potential as a target for treating metastatic disease.
TGF-β Signaling in Myeloid Cells Is Required for Tumor Metastasis
This article was highlighted IN THE SPOTLIGHT of Cancer Discovery: Myeloid TGF-β Responsiveness Promotes Metastases
Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-β signaling
This article was featured as a Research Highlight in Nature Reviews Cancer: Microenvironment: Making connections
Lab Life