
Vinay K. Pathak, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 535, Room 334
- Frederick, MD 21702-1201
- 301-846-1710
- pathakv@mail.nih.gov
RESEARCH SUMMARY
Dr. Pathak has significantly advanced the field of lentiviral molecular virology with his studies of HIV-1 replication in infected cells. Under his direction, the Viral Mutation Section has developed innovative live-cell microscopy methods to show that, in contrast to most HIV-1 replication models, intact viral cores are transported into the nucleus, complete reverse transcription in the nucleus, and disassemble (uncoat) near their integration sites just before integration. Dr. Pathak’s group has significantly contributed to our understanding of how HIV-1 replicates in the presence of potent host restriction APOBEC3 proteins and antiviral drugs. Additionally, Dr. Pathak’s group played a key role in discovering the origin of a newly identified retrovirus, XMRV, and in quelling a potential public health crisis by refuting the controversial claims associating this virus with chronic fatigue syndrome and prostate cancer.
Areas of Expertise

Vinay K. Pathak, Ph.D.
Research
HIV-1 Replication, Host Restriction Factors, Antiviral Drug Resistance, and Development of Novel Therapeutics
Project 1. Elucidate HIV-1 replication in infected cells by high-resolution live-cell microscopy. Many aspects of HIV-1 replication in infected cells, including nuclear import and capsid dissembly, which are essential for HIV-1 replication, remain poorly understood. To gain insights into these essential steps during HIV-1 replication in infected cells, we developed a new method to label viral complexes by fluorescently tagging APOBEC3F, which is incorporated into virions. Using this method, we showed that reverse transcription is not required for nuclear import of viral complexes, and that HIV-1 capsid interactions with host proteins are essential for nuclear import of HIV-1 capsids (Burdick et al., PNAS 2013). The dynamics and regulation of HIV-1 nuclear import and its intranuclear movements after import had not been previously studied. We carried out high-resolution live-cell microscopy studies of APOBEC3F-labeled viral complexes to determine the dynamics of HIV-1 nuclear envelope docking, nuclear import, and intranuclear movements by live-cell microscopy (Burdick et al., PLoS Pathog. 2017). For several decades, retroviral core uncoating has been thought to occur in the cytoplasm in coordination with reverse transcription or at the nuclear envelope during nuclear import, but no studies have concluded that uncoating occurs in the nucleus. We developed methods to study HIV-1 uncoating by direct labeling and quantification of viral capsid protein associated with infectious capsids. Our results show that intact (or nearly intact) viral cores enter the nucleus through a mechanism involving interactions with host protein cleavage and polyadenylation specificity factor 6 (CPSF6), complete reverse transcription in the nucleus before uncoating, and uncoat less than 1.5 hours before integration near their genomic integration sites. These results fundamentally change our current understanding of HIV-1 post-entry replication events including mechanisms of nuclear import, uncoating, reverse transcription, integration, and evasion of innate immunity (Burdick et al., PNAS 2020).
Project 2. Elucidate the structure and function of APOBEC3 proteins and develop new strategies to treat and functionally cure HIV-1 infection. Our goal is to understand the structure and function of human restriction factor APOBEC3 proteins, which are an important innate immune defense mechanism that protects against invading pathogens. APOBEC3 proteins potently inhibit HIV-1 replication in the absence of the virally encoded Vif protein by incorporating into virions in the virus producing cells and inducing lethal G-to-A hypermutation of the viral DNA in infected target cells. Vif overcomes these host defenses by targeting the APOBEC3 proteins for proteasomal degradation. We have significantly contributed to elucidating the APOBEC3 and Vif determinants that interact with each other (Xu et al., PNAS 2004; Russell & Pathak, J. Virol. 2007; Smith & Pathak, J. Virol. 2010). We have shown that A3G and A3F both inhibit viral DNA integration, but through distinctly different mechanisms (Mbisa et al., J. Virol. 2007; Mbisa et al., J. Virol. 2010). We also developed a bimolecular fluorescence complementation assay to visualize A3G and A3F molecular interactions in living cells and observed that A3G and A3F multimerization, A3G-Gag interactions, and A3G virion incorporation require RNA binding (Friew et al., Retrovirology 2009). We compared the antiviral activity of A3G and A3F in primary CD4+ T cells and macrophages, the natural target cells of infection, and found that while both can inhibit HIV-1 replication A3G is a more potent inhibitor of HIV-1 than A3F (Chaipan et al., J. Virol. 2013).
Recently, in collaboration with Dr. Yong Xiong (Yale University), we determined the structure of a Vif-APOBEC3F-CTD-CBFb complex (Hu et al., Nat. Struct. Mol. Biol. 2020). We demonstrated that APOBEC3 proteins potently induce lethal hypermutation and contribute minimally to viral genetic variation (Delviks-Frankenberry et al., PLoS Pathog 2016). We are developing lentiviral vectors that can efficiently deliver the Vif1-resistant APOBEC3G to hematopoietic stem cells, with the goal of providing a proof of concept that delivering Vif-resistant APOBEC3G to infected patients can provide an approach for a functional cure for HIV-1 infection (Delviks-Frankenberry et al., Mol. Ther. Nucleic Acids 2019).
Project 3. Define mechanisms that confer resistance to nucleoside and nonnucleoside reverse transcriptase (RT) inhibitors. Based on our previous observations on RT template switching and recombination, we proposed that the relative polymerase and RNase H activities of RT can affect the balance between RNA degradation and nucleotide excision, and that mutations that reduce RNase H activities can enhance resistance to nucleoside as well as nonnucleoside RT inhibitors. We observed that several mutations that reduce RNase H activity affect the balance between RNA degradation and nucleotide excision and enhance nucleoside RT inhibitor resistance (Nikolenko et al., PNAS 2005; Nikolenko et al., PNAS 2007; Delviks-Frankenberry et al., PNAS 2008). Our studies have demonstrated that mutations in the connection and RNase H domains also increase resistance to nonnucleoside RT inhibitors, and suggest a parallel mechanism by which resistance to both classes of RT inhibitors can be increased (Nikolenko et al., J. Virol. 2010). These hypotheses were validated by characterization of RT mutations observed in patients and subsequently confirmed by other labs.
Project 4. Demonstrate that xenotropic murine leukemia virus-related virus (XMRV) is a laboratory recombinant and not a human pathogen. XMRV, a gammaretrovirus, was reportedly found in prostate cancer and chronic fatigue syndrome (CFS) patients at high frequencies and thought to be a human pathogen. However, we found that XMRV replication is severely restricted by human APOBEC3 proteins (Paprotka et al., J. Virol. 2010), and its replication is severely restricted in human peripheral blood mononuclear cells, raising doubts about its pathogenicity in humans (Chaipan et al., J. Virol. 2011). To gain insights into the origin of XMRV, we analyzed prostate cancer xenografts that were passaged in nude mice and used to develop a XMRV-positive human cancer cell line. The results showed that XMRV is most likely a recombinant between two murine endogenous proviruses that we named PreXMRV-1 and PreXMRV-2 (Paprotka et al., Science 2011). We concluded that the association between XMRV and human disease is due to contamination of human samples with a laboratory recombinant virus.
Pathak Lab, March 2022
Publications
- Bibliography Link
- View Dr. Pathak's PubMed Summary
- View Dr. Pathak's ORCID Bibliography
HIV-1 uncoats in the nucleus near sites of integration (article awarded 2020 Cozzarelli Prize)
Dynamics and regulation of nuclear import and nuclear movements of HIV-1 complexes
Minimal contribution of APOBEC3-induced G-to-A hypermutation to HIV-1 recombination and genetic variation
Recombinant origin of the retrovirus XMRV
Biography

Vinay K. Pathak, Ph.D.
Dr. Vinay K. Pathak received his B.A. in Biology from the University of California, Los Angeles, in 1979. He obtained his M.S. in Comparative Pathology in 1983 from the University of California, Davis, for characterization of mouse mammary tumor virus proviral integration sites near the int1 and int2 loci in mammary tumors and hyperplastic tissues in Dr. Robert Cardiff's laboratory. He received his Ph.D. for work on characterization of the eukaryotic protein synthesis initiation factors eIF-2α and eIF-2β in Dr. John W.B. Hershey's laboratory at the University of California, Davis, in 1988. He was a postdoctoral fellow under the guidance of Dr. Howard Temin from 1988 to 1991, where he determined the in vivo forward mutation rate of spleen necrosis virus and characterized the nature of mutations that arise during retroviral replication. These studies were the first to report G-to-A hypermutation in a retrovirus, now known to be the result of cytidine deamination by host restriction APOBEC3 proteins. Dr. Pathak became an Assistant Professor in the Department of Biochemistry and the Mary Babb Randolph Cancer Center at West Virginia University in 1991 and was promoted to Associate Professor with tenure in 1998. He joined the National Cancer Institute in 1999 as Senior Investigator and Head of the Viral Mutation Section in the HIV Dynamics and Replication Program (renamed the HIV Dynamics and Replication Program in 2015). He was appointed as Guest Editor for the "HIV Drug Resistance" special issue of Viruses, published in 2010. He received the NIH Asian and Pacific Islander American Organization Award for outstanding accomplishments in biomedical research and an NIH Group Merit Award in 2012 and an NIH Special Act or Service Award in 2020. A research team led by Dr. Pathak received the 2020 Cozzarelli Prize in the Biomedical Sciences class for their PNAS publication “HIV-1 uncoats in the nucleus near sites of integration”; this prize was awarded to just 6 papers chosen from nearly 4,000 published research articles in 2020, recognizing the most outstanding contributions to the scientific disciplines represented by the National Academy of Sciences. Dr. Pathak has also received numerous research funding awards, including a U.S.-Russian Joint Working Group on Biomedical Research Cooperation Award, six NIH Intramural AIDS Targeted Antiviral Program (IATAP) Research Awards, eight IATAP Equipment Awards, two NIH Office of AIDS Research (OAR) Innovation Awards, an OAR Intramural Project Award, and two NIH Bench-to-Bedside Awards. He served as Co-Chair (2013-2014) and Chair (2015) of the Annual Norman P. Salzman Memorial Symposium in Virology, NIH. In 2017, he served as Co-Organizer of the Cold Spring Harbor Laboratory Retroviruses Meeting. He was Co-Organizer of the widely attended NIH/FDA COVID-19 Research Symposium/Workshop in 2020 and is one of six Associate Chairs and the sole NCI member of the NIH-wide COVID-19 Scientific Interest Group Leadership Committee. He also currently serves as a member of the NCI RNA Biology Initiative and the Editorial Boards of Retrovirology, Journal of Virology, and Viruses.
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
NIH Fellows Awards for Research Excellence
Sushila Kumari received a 2026 NIH Fellows Award for Research Excellence (FARE) for travel to attend and present her work at a scientific meeting in the U.S. This award, which acknowledges outstanding scientific research performed by intramural postdoctoral fellows, is sponsored by the NIH Fellows Committee, Scientific Directors, and Office of Intramural Training and Education and is funded by the Scientific Directors. FARE awards are based on scientific merit, originality, experimental design, and overall quality and presentation of the abstracts.
Members of the Pathak lab who were FARE awardees in previous years include Sushila Kumari (2022, 2024), Chenglei Li (2021), Mohamed Husen Munshi (2020), Sanath Kumar Janaka (2017), Tobias Paprotka (2012), Narasimhan Jayanth Venkatachari (2012), Wei Bu (2010), Jessica Smith (2010), Rebecca Russell (2009), Krista Delviks-Frankenberry (2008), Yeshitila Friew (2007), Patricia Henry (2007), and Galina Nikolenko (2007).
Pathak lab at CSHL Retrovirology Meeting 2025
Members of the Pathak lab attended the 2025 Cold Spring Harbor Laboratory Retrovirology Meeting.
Sushila Kumari and Ryan Burdick were selected to give oral presentations. Akshay Deshpande and Ellie Bare gave a poster presentations.
Krista Delviks-Frankenberry publishes in J. Virology
In collaboration with Wei-Shau Hu's lab, Krista Delviks-Frankenberry and Vinay Pathak publish in the journal Virology.
- How to recover from a bad start: adaptation of HIV-1 transcription start site mutations during serial passaging in culture. J. Virol May 7;e0015925.
Travel Awards, HIV DRP Think Tank Meeting
Ryan Burdick received a $1000 travel award from the HIV DRP for one of the two best presentations by NCI fellows at the 2025 HIV DRP Think Tank Meetings.
Vinay Pathak publishes in Viruses
In collaboration with Wei-Shau Hu's lab, Vinay Pathak publishes in Viruses showing that elements in the 5' untranslated region of viral RNA important for HIV gag recognition and cross-packaging (2025).
- Elements in the 5' Untranslated Region of Viral RNA Important for HIV Gag Recognition and Cross-Packaging. Viruses. April 10; 17(4):552.
Vinay Pathak publishes in mBio
In collaboration with Wei-Shau Hu's lab, Vinay Pathak publishes in the journal mBio, showing HIV-1 transcription start sites usage and its impact on unspliced RNA functions in people living with HIV (2024). This work was also done in collaboration with Frank Maldarelli and Robert Gorelick (FNL).
- HIV-1 transcription start sites usage and its impact on unspliced RNA functions in people living with HIV. mBio Dec 27:e0357624.
Poster Presentations at the NIH HIV Structural Biology Meeting 2024
Ryan Burdick (biologist) and Chenglei Li (post-doc) presented posters at the 2024 HIV Structural Biology Meeting at NIH-Bethesda. A VideoCast is available here.
Vinay Pathak publishes in J Virol
In collaboration with Wei-Shau's lab, Vinay Pathak publish in the journal J Virol, showing translation of HIV-1 unspliced RNA is regulated by 5' untranslated region structure (2024). This work was also done in collaboration with Ohio State University.
- Translation of HIV-1 unspliced RNA is regulated by 5' untranslated region structure. Oct 22;98(10):e0116024.
Krista Delviks-Frankenberry publishes in Nature Structural Molecular Biology
In collaboration with Yale University, Krista Delviks-Frankenberry publishes in the journal Nature Structural Molecular Biology, with her paper titled "Structural insights into PPP2R5A degradation by HIV-1 Vif" (2024).
- Structural insights into PPP2R5A degradation by HIV-1 Vif. Nat. Struct. Mol. Biol. 2024. May 24. doi: 10.1038/s41594-024-01314-6.
Pathak lab at CSHL Retrovirology Meeting 2024
Members of the Pathak lab attended the 2024 Cold Spring Harbor Laboratory Retrovirology Meeting.
Ryan C. Burdick (biologist) delivered his talk as the recipient of the 2024 Uta Von Schwedler Award. Poster presentations were made by Ellie Bare (post-doc) and Chenglei Li (post-doc).
Intramural AIDS Research Fellowships
Intramural AIDS Research Fellowship (IARF) awards from the Office of AIDS Research, Office of Intramural Research, and Office of Intramural Research & Training in the National Institutes of Health include full stipend support to successful candidates who demonstrate outstanding scientific potential through both an imaginative and thoughtful research plan and a well thought out career development plan.
Sushila Kumari received an IARF award in 2024 to support her research project.
The following postdoctoral fellows in the Pathak lab received IARF awards in previous years:
Rokeya Siddiqui received an IARF award in 2022 and 2021 to support her research.
Belete Desimmie: "Identification of Novel Class of HIV Replication Inhibitors Targeting the HIV-1 Vif-A3G Interactions" (2014)
Narasimhan Jayanth Venkatachari: "Identification of Small Molecule Inhibitors of Vif-A3G and Vif-A3F Interactions as Novel Antiviral Agents for the Treatment of HIV-1 Infection" (2010, 2011)
Ryan Burdick receives the 2024 Uta von Schwedler Prize for Retrovirology
Congratulations to Ryan Burdick for winning the 2024 Uta von Schwedler Prize.
The award was given to Ryan Burdick for his numerous contributions to our understanding of HIV-1 uncoating. Ryan’s most recent paper, “HIV-1 uncoating requires reverse transcription of long double-stranded DNA” is now published in Science Advances.
The Uta von Schwedler Prize for Retrovirology was established in 2012 to annually award and honor the accomplishments of a distinguished scientist in retrovirology. Ryan will receive a $1250 cash prize and present a short talk and poster at this year’s Retroviruses meeting at Cold Spring Harbor Laboratories.
Vinay Pathak talks at The Palm Springs Symposium On HIV/AIDS
Vinay Pathak was an invited speaker at The Palm Springs Symposium On HIV/AIDS 2024. The title of his talk was "HIV-1 Uncoating Requires Synthesis of Long Reverse Transcription Products".
Travel Awards, Fall HIV/AIDS & Cancer Virology Think Tank Meeting
Ryan Burdick won a $1200 travel award for his outstanding oral presentation at the 2023 Fall HIV/AIDS & Cancer Virology Think Tank Meeting. In addition Rokeya Siddiqui won a $800 travel award for her outstanding poster presentation at the 2023 Fall HIV/AIDS & Cancer Virology Think Tank Meeting. This annual Think Tank meeting on the NIH-Bethesda campus provides a venue for students, postdoctoral fellows, and staff scientists to present emerging work and hypotheses in the field of cancer virology. The Think Tank travel awards are provided by the Center of Excellence in HIV/AIDS & Cancer Virology, Center for Cancer Research, NCI. Previous winner from the Pathak lab: Krista Frankenberry (2022).
New Investigator Scholarships, Conference on Retroviruses and Opportunistic Infections
Chenglei Li was awarded a New Investigator Scholarship to present his research findings in the 2021 Conference on Retroviruses and Opportunistic Infections (CROI). CROI scholarship awardees in previous years include Mohamed Husen Munshi and Chenglei Li (2020), Mohamed Husen Munshi (2019), Belete Desimmie (2017), and Taisuke Izumi (2013).
Alumni
Covers
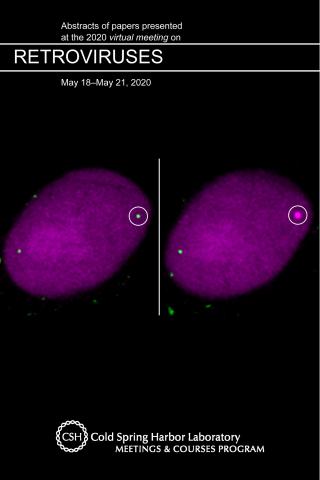
HIV-1 Uncoats in the Nucleus Near Sites of Integration
A recent study published by the research groups of Vinay Pathak and Wei-Shau Hu in Proceedings of the National Academy of Sciences USA (“HIV-1 uncoats in the nucleus near sites of integration,” PNAS 117:5486-5493, 2020) was featured on the front cover of the Cold Spring Harbor Laboratory 2020 Retroviruses Meeting abstract book. Pathak lab member Ryan Burdick launched the meeting with his talk on the study and reported that HIV-1 uncoats in the nucleus near sites of integration. Contrary to the prevailing theory for more than 40 years that retroviral uncoating occurs in the cytoplasm, the study team showed that HIV-1 cores are essentially intact as they enter the nucleus, where they complete reverse transcription before uncoating near their sites of integration into the host genome. These unexpected results fundamentally alter the current understanding of HIV-1 replication, which could lead to the development of more effective strategies and drugs for the treatment of HIV infections.
In the cover image, the left panel shows an HIV-1 capsid localized in the nucleus and the right panel shows a site of transcription of the viral genome at the site where the capsid localized.
To read more about the study, see the original research article and the commentary "Entering and breaking for HIV?" in Nature Reviews Microbiology.
This study was also awarded the 2020 PNAS Cozzarelli Prize in Biomedical Sciences and featured on the website of the Center for Cancer Research, National Cancer Institute (“New study overturns conventional understanding of how HIV infection occurs”).
Burdick RC, Li C, Munshi MH, Rawson, Nagashima K, Hu W-S, Pathak VK. Cold Spring Harbor Laboratory Retroviruses Meeting Abstract Book, May 2020.










