Vanja Lazarevic, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 10 - Magnuson CC, Room 5A31/33
- Bethesda, MD 20892
- 240-858-3344
- vanja.lazarevic@nih.gov
RESEARCH SUMMARY
Our laboratory investigates the dynamic immune cell interactions in the meninges and the central nervous system (CNS). Utilizing a multidisciplinary approach, our research aims to mechanistically understand how transcription factors influence the behavior and adaptation of innate lymphoid cells (ILCs) and CD4+ T helper cells within the CNS environment. Our objective is to pinpoint molecular pathways in ILCs and T cells that are key drivers of neuroinflammation and neurodegeneration.
Areas of Expertise

Vanja Lazarevic, Ph.D.
Research
Our laboratory investigates the dynamic immune cell interactions in the meninges and the central nervous system (CNS). Utilizing a multidisciplinary approach, our research aims to mechanistically understand how transcription factors influence the behavior and adaptation of innate lymphoid cells (ILCs) and CD4+ T helper cells within the CNS environment. Our objective is to pinpoint molecular pathways in ILCs and T cells that are key drivers of neuroinflammation and neurodegeneration.
Current projects:
Transcriptional regulation of CD4+ T cell responses in autoimmunity: This project examines the molecular underpinnings governing the developmental plasticity and tissue adaptation of CD4+ T helper cells. Utilizing the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis, we focus on identifying key transcription factors and their gene targets that orchestrate inflammatory responses in the CNS.
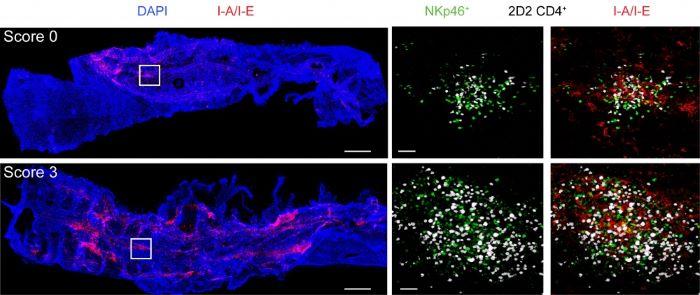
Immunoregulatory role of ILCs in the CNS: The CNS is widely recognized as an immune-privileged site that tightly regulates interactions with immune cells to protect neurons from the detrimental effects of inflammation. Nevertheless, the meninges and associated CNS bone marrow niches are populated with a diverse array of immune cells engaged in ongoing immunosurveillance. Despite this immune presence, the CNS borders serve as tightly regulated gateways, selectively controlling immune cell entry into the CNS parenchyma. Our research seeks to understand how these immunological interactions at the CNS borders translate into broader immune responses in the context of CNS autoimmunity. Our previous studies have shown that T-BET-dependent NKp46+ ILCs play a critical role in regulating adaptive immune responses within the meninges by affecting the optimal reactivation and stability of myelin-reactive CD4+ Th17 cells. This project is focused on delineating the functions and regulatory mechanisms of ILCs, particularly those within the meninges. We explore their tissue origin and how these meningeal ILCs uphold CNS integrity and their role in triggering or propagating neuroinflammatory diseases.
Deciphering the molecular basis of disrupted self-tolerance in autoimmunity: Autoimmune diseases such as multiple sclerosis manifest when the immune system erroneously attacks healthy tissues, a process often precipitated by the failure of mechanisms designed to distinguish 'self' from 'non-self.' This project seeks to unravel the molecular foundations of this breakdown in self-tolerance, focusing on T cell receptor (TCR) signaling. Our goal is to map how tissue-specific signals and inflammatory cytokines alter TCR signaling, contributing to the onset of CNS autoimmune disease.
Can we enhance anti-tumor immunity against glioblastoma multiforme (GBM)? GBM presents a critical challenge due to its aggressive nature and resistance to existing treatments. This project involves a comprehensive examination of the immune microenvironment within GBM, aiming to decode the complex interactions between immune cells, cancer cells, and neurons. Our goal is to uncover how these interactions can be manipulated to bolster anti-cancer immunity and dampen cancer-promoting inflammation, opening new avenues for therapeutic intervention.
Publications
Transcription factor EGR2 controls homing and pathogenicity of TH17 cells in the central nervous system
T-bet-dependent NKp46+ innate lymphoid cells regulate the onset of TH17-induced neuroinflammation
The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells
T-bet represses TH17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt
Biography

Vanja Lazarevic, Ph.D.
B.Sc., Microbiology, University of Nottingham, Nottingham, UK
Ph.D., Molecular Virology and Microbiology, University of Pittsburgh, Pittsburgh, PA
Postdoctoral Fellow, Immunology Senior Research Associate, Laboratory of Dr. Laurie H. Glimcher, Harvard School of Public Health, Boston, MA
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
News from Our Lab
T-bet-dependent NKp46(+) innate lymphoid cells regulate the onset of T(H)17-induced neuroinflammation. Nature Immunology, 18(10): 1117-1127, 2017 (DOI: 10.3410/f.728639681.793538742), has been recommended in F1000Prime as being of special significance in its field.
PubMed Abstract Free PMC Article
Covers

T-bet-dependent NKp46+ innate lymphoid cells regulate the onset of TH17-induced neuroinflammation.
The process by which self-reactive CD4+ T cells infiltrate the central nervous system (CNS) and trigger neuroinflammation is not fully understood. Lazarevic and colleagues show that NKp46+innate lymphoid cells dependent on the transcription factor T-bet are critical mediators in facilitating the entry of autoreactive CD4+ cells of the TH17 subset of helper T cells into the CNS, which leads to autoimmunity. Artwork by Lewis Long.
Original article: Nature Immunology 2017 Oct;18(10):1117-1127. doi:10.1038/ni.3816.
News and Views: Nature Immunology 2017 Oct;18(10):1063-1064. doi:10.1038/ni.3839
Lab Life