Mark R. Gilbert, M.D.
- Center for Cancer Research
- National Cancer Institute
RESEARCH SUMMARY
As the former Neuro-Oncology Branch (NOB) chief from 2014 to 2024, Dr. Gilbert developed a unified team to perform cutting-edge research that advances knowledge in the brain tumor field. These findings were used to develop robust clinical trials, which improved patient outcomes through innovative combination treatments and precision medicine approaches.
Today, Dr. Gilbert is a scientist emeritus. In his previous role as chief and senior investigator, he led the NOB's Translational Immunology Research Program and Precision Medicine Research Program. His primary research concentrations were in the areas of immunotherapy and precision therapy. For immunotherapy, he and his team worked to develop combination therapies that increase immune cell recruitment to the tumor site and improve patient selection in clinical trials to maximize benefit for those enrolled. His Precision Medicine Research Program focused on utilizing basic and computational biology to develop synthetic lethal drug pairs that can overcome the challenges of driver mutation targeting—offering a wider percentage of patients effective therapeutic options.
Explore the NOB's Research Programs >
Areas of Expertise
Research
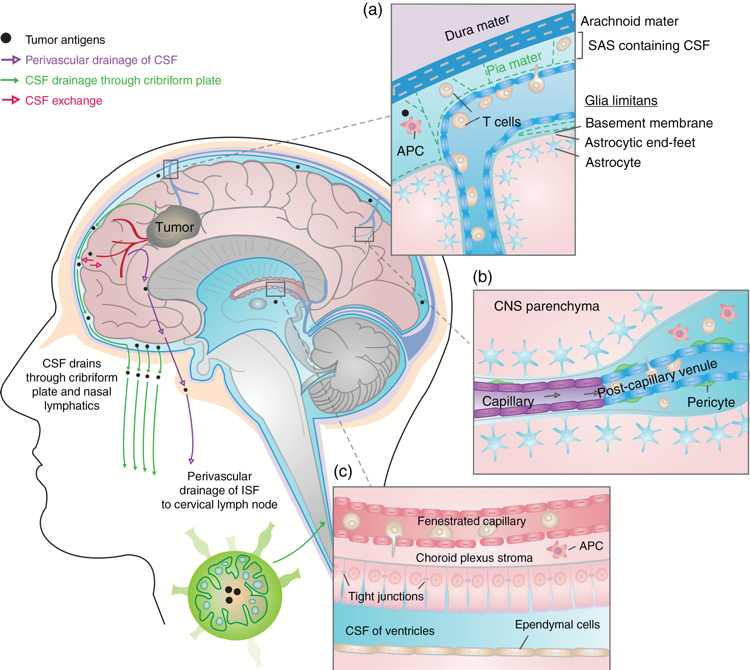
Advances in therapeutic approaches for patients with primary brain tumors have struggled to keep pace over the last few decades, as surgery, radiation, and chemotherapy remain the staples of clinical care. In his previous role as a senior investigator at the Neuro-Oncology Branch (NOB), Dr. Gilbert established a robust Translational Immunology Research Program with a multidisciplinary research group to improve treatment and patient outcomes. Dr. Gilbert’s vision was to not only find effective treatments for brain tumors, but to also establish paradigms of clinical and translational investigation that can then be utilized worldwide to help other physicians make a collective impact in neuro-oncology.
Because heterogeneity is a hallmark of brain tumors, Dr. Gilbert’s focus was exploring precision medicine and immunotherapy options by utilizing computational, biological, and clinical trial approaches stemming from a strong basic research program. His roles in founding and establishing the Brain Tumor Trials Collaborative (BTTC) and the Collaborative Ependymoma Research Network (CERN) were crucial foundational pillars to establish a hypothesis-driven research program that supported pre-clinical and clinical studies to advance therapies for rare cancers. The BTTC and CERN are now part of large, multi-institutional networks that focus on delivering clinical trial options for malignant gliomas around the country, supported by molecular profiling, patient outcomes studies, and innovative drug discovery, among other goals.
Publications
- Bibliography Link
- View Dr. Gilbert's PubMed Summary
A Phase II Study of Dose-Dense Temozolomide and Lapatiniv for Recurrent Low-Grade and Anaplastic Supratentorial, Infratenrial, and Spinal Cord Ependymoma
Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy.
Randomized phase II adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma.
A randomized trial of bevacizumab for newly diagnosed glioblastoma
Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial
Biography

Mark R. Gilbert, M.D.
Dr. Gilbert retired as chief of the Neuro-Oncology Branch (NOB) in May 2024 and is now an NIH scientist emeritus.
He received his Doctor of Medicine degree from Johns Hopkins University in 1982 (Alpha Omega Alpha). He remained at Johns Hopkins to complete residencies in internal medicine (1982-1985), neurology (1984-1988), and neuro-oncology fellowship training with the Keck Foundation Fellowship (1986-1987).
He subsequently held academic research positions at Johns Hopkins University’s Department of Neurology and Oncology Center, the University of Pittsburgh Cancer Institute’s Department of Neurology, and Emory University’s Department of Neurosurgery. He then had a 14-year tenure at the Department of Neuro-Oncology at The University of Texas’s MD Anderson Cancer Center before becoming chief of the NOB in 2014.
Over the course of his career, Dr. Gilbert has been an integral part of various national and international clinical trial initiatives. Beginning in 2005, he was appointed director of the Brain Tumor Trials Collaborative (BTTC), a multi-center clinical trials consortium that is a large part of the NOB’s ongoing efforts to deliver accessible clinical trial options to patients across the country. Additionally, Dr. Gilbert is the founder, former director, and principal investigator of the Collaborative Ependymoma Research Network (CERN), a consortium formed in 2006 to study ependymoma—a rare central nervous system cancer. CERN supports basic research, clinical trials, patient outcomes research, and educational efforts in North America and Europe.
Notably, Dr. Gilbert received the Blanche Bender Endowed Professorship in Cancer Research in 2009 from the MD Anderson Cancer Center, and the National Institutes of Health Director’s Award in 2016 for his impactful contributions to the field.
Honors, Awards and Leadership
- Top Doctors - 2002-Present
- Best Doctors - 2002-Present
- Joel A. Gingras Jr. Award, American Brain Tumor Association - 2022
- Federal Technology Transfer Award, Cancer Center Research - 2016-2020
- National Institute of Health Director’s Award - 2016
- Award for Excellence in Clinical Research, Society for Neuro-Oncology - 2010, 2011, 2013
- Matthew T. Moore Distinguished Lecturer - 2010
- Blanche Bender Endowed Professorship in Cancer Research - 2009
- Who's Who in America - 2006
- Vice President, Society for Neuro-Oncology - 2005-2007
- Phi Beta Kappa, The Johns Hopkins University - 1982
- Alpha Omega Alpha, The Johns Hopkins University - 1982
- Scholars Program, Department of Chemistry, Brooklyn College - 1976
Societies and Initiatives
- Founder, Former Director and Principal Investigator, Collaborative Ependymoma Research Network (CERN)
- Director, Brain Tumor Trials Collaborative (BTTC)
Professional Memberships
- Brain Tumor Trials Collaborative (BTTC)
- Collaborative Ependymoma Research Network (CERN)
- Radiation Therapy Oncology Group (RTOG)
- Society for Neuro-Oncology (SNO) Foundation Board
- Adult Brain Tumor Consortium (ABTC)
- American Academy of Neurology (AAN)
- American Association for Cancer Research (AACR)
- New Approaches to Brain Tumor Treatment Consortium (NABTT)
News
Forming Connections to Address Rare Brain Tumor Treatment and Care
May 21, 2024
Scientists and neuro-oncology providers are taking a comprehensive approach to improve care and treatment—performing laboratory research, conducting clinical trials, and fostering a sense of community. Watch Now >
Celebrating CCR Careers: Mark Gilbert, M.D.
April 23, 2024
After nearly a decade at the Center for Cancer Research as chief of the Neuro-Oncology Branch, Dr. Mark Gilbert is announcing his retirement. Read more >
January 30, 2024
In a new systematic review, researchers identify the specific challenges that make it difficult for this patient population to enroll in clinical trials and seek specialized treatment. Read more >
Mark Gilbert, M.D., Earns American Brain Tumor Association’s Joel A. Gingras Jr. Award
September 5, 2022
The Neuro-Oncology Branch chief was recognized for his work spearheading brain tumor research collaborations and expanding clinical trial access. Read more >
August 11, 2022
Summer interns investigated methods to improve immunotherapy, tailor survivorship programs, and target cancer cells using antibiotics. Read more >
Translational Immunology: Making Immunotherapy Effective for Patients with Brain Tumors
Dr. Mark Gilbert leads the Translational Immunology Program, which investigates the unique nature of the immune response in the brain to improve patient outcomes. Read more >
New Immunotherapy Study for Glioblastoma
A clinical trial investigates immune checkpoint inhibitors with standard treatment in people with aggressive brain cancers to understand the immune system response and improve long-term outcomes. Read more >