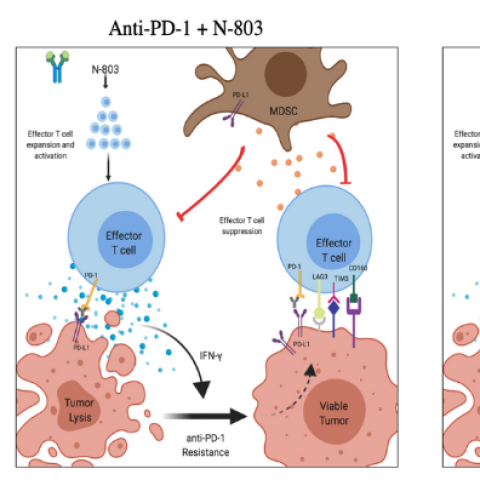
Triplet therapy with PD-L1 CAR-NK + anti-PD-1 + N-803 is hypothesized to provide multiple immune enhancements and addresses multiple immune deficits in the anti-tumor response.
Image credit: Dr. Jason Redman, made in BioRender
Adults with gastric or head and neck cancer may be eligible to participate in a clinical trial at the NIH Clinical Center.
Jason M. Redman, M.D., Assistant Research Physician in the Genitourinary Malignancies Branch, is leading this study that researches if several types of immunotherapy – treatment that uses the body’s own immune system to fight cancer – can shrink gastric or head and neck tumors.
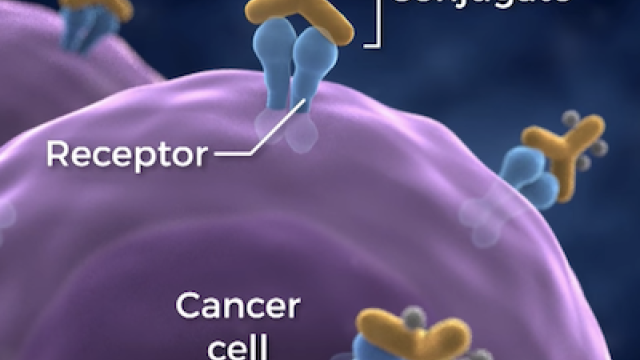
Researchers are using three types of immunotherapy in this trial, including a novel natural killer (NK)-cell therapy. NK cells are an important part of the immune system’s ability to fight cancer. The NK cells used in this study are engineered to recognize and kill cells that have a protein called PD-L1 on their surfaces. PD-L1 can be expressed on cancer cells but also on other cells that help the cancer avoid the immune system. These NK cells can kill the cancer cells and the cells that aid the cancer.
Trial participants will receive the engineered NK cells in combination with pembrolizumab, an immunotherapy drug that blocks tumor cells’ ability to escape the immune system, and a drug called N-803. N-803 is an immunotherapy that mimics the activity of interleukin-15, a substance that supercharges cancer-fighting parts of the immune system.
Clinicaltrials.gov identifier: NCT04847466
NCI Protocol ID: NCI-00-0-096
Official Title: Immunotherapy Combination: Irradiated PD-L1 CAR-NK Cells Plus Pembrolizumab Plus N-803 for Subjects With Recurrent/Metastatic Gastric or Head and Neck Cancer
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.