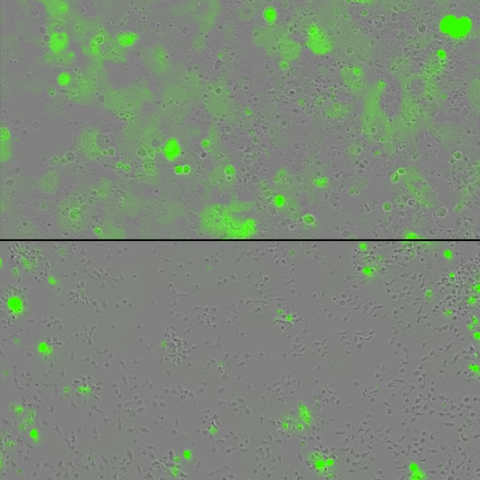
Tumor cells (green) are grown in a dish with engineered T cells alone (top) or with engineered T cells harboring two cytokines tethered to their surface (bottom). The engineered T cells containing the tethered cytokines lead to greater eradication of tumors. Image credit: Rosa Nguyen, M.D., Ph.D.
Researchers from CCR have developed a new technology to help T-cell therapies work better against solid tumors using mice as models of adult and pediatric cancers. The advance, published November 1, 2023, in Clinical Cancer Research, introduces a novel approach that allows immune cells to overcome the challenging environment inside those tumors.
Autologous T-cell therapy is a type of immunotherapy in which a patient’s own immune cells are removed from the body, manipulated in the lab to better fight cancer cells, and multiplied before being given back to the patient by intravenous infusion. While T-cell therapy has been successful in treating many blood cancers, the same success has not been reached for a large number of solid tumors.
This resistance to T-cell therapy partly stems from solid tumor bulkiness and confinement, said Rosa Nguyen, M.D., Ph.D., Lasker Clinical Research Scholar in the Pediatric Oncology Branch. “Solid tumors lack the optimal environment for T cells to function,” she continued, “because their surroundings lack the nutrients and signals needed to help stimulate and support T-cell activity.”
Among these missing signal components are molecules called cytokines, Nguyen explained, which help initiate and amplify T-cell activity. To address this problem, Nguyen and her team, in collaboration with her CCR colleagues and scientists from Rutgers Cancer Institute, New Brunswick, New Jersey, developed a method to attach two important cytokines to the surface of engineered T cells. Now, not only would the T cells seek out cancer, but in doing so they would carry their own cytokines into the tumors to support tumor cell destruction.
In a series of experiments, the team looked to see if the technology could help T cells overcome exhaustion when dealing with a particularly large tumor load. They grew their modified T cells together with cancer cells in a dish, and as the T cells eradicated the cancer, the researchers continued to add new cancer cells to the mix. Unlike T cells without the two cytokines, the technology showed fewer signs of exhaustion, and the engineered T cells had an enhanced tumor-fighting capacity, Nguyen said.
Next, to validate their findings in the dish, Nguyen’s team used the engineered T cells to treat mouse models of adult cervical cancer and pediatric neuroblastoma. In addition, they tested two types of T-cell therapies that are currently used in clinical trials to treat cancers in people.
In all combinations tested, the mice treated with engineered T cells harboring the two cytokines had the best tumor clearance and lived the longest. Notably, in the adult cervical cancer mouse model, the new technology eradicated the tumors completely in 80 percent of the mice. Although cytokines can sometimes overstimulate the immune system to ill effect, the experimental treatment did not cause any serious side effects in these two models, Nguyen noted.
Nguyen’s team is continuing to test the T-cell therapy technology in mouse models of additional cancers and with various types of engineered T cells. With more data from these experiments in mice, Nguyen hopes to move this treatment into clinical trials to enhance the effectiveness of T-cell therapy for patients with solid tumors.