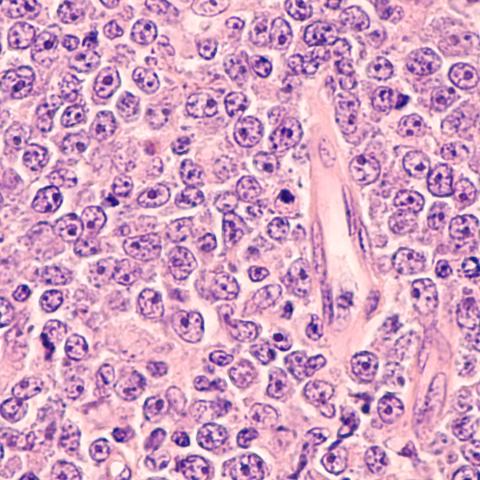
Photomicrograph of a diffuse large B-cell lymphoma (DLBCL).
Image credit: rightdx/iStock
Researchers at CCR have made progress in identifying a pre-cancerous cell-of-origin for an aggressive subtype of lymphoma. The results, published June 27 in Blood, could help scientists better study the MCD/C5 genetic subclass of diffuse large B cell lymphoma (DLBCL) and identify more effective therapies for this disease.
DLBCL is a type of cancer that arises from specialized immune cells called B cells, which make antibodies that are important for defending our bodies from harmful bacteria or viruses. B cells make antibodies by entering specialized structures, called germinal centers, where they can mutate their own genes that are responsible for antibody production. By doing so, B cells can create unique and tailored antibodies that target specific pathogens. Although germinal centers most often form in response to immunization or infection, they can also form spontaneously in immune organs such as the spleen.
“One of the costs or trade-offs with actively mutated antibody genes is that you can get mutations in other genes too, which may predispose a person to develop DLBCL,” explains Jagan Muppidi, M.D., Ph.D., a Stadtman Investigator in CCR’s Lymphoid Malignancies Branch who studies the implications of these mutations.
In this study, Muppidi and his colleagues investigated how genetic mutations associated with one particularly aggressive subtype of DLBCL, called MCD, affected B cell immune response in mice. They discovered that the combined expression of four mutations — which include giving new abilities to the Myd88 and Cd79b genes, inactivity of the Prdm1 gene and overexpression of the BCL2 gene — caused precancerous B cell expansions to form specifically in spontaneous germinal centers in the animals’ spleens. About half of these mice went on to develop DLBCL later in their life.
“We found that B cells in germinal centers that form spontaneously in the spleen are likely to be the cells of origin for this disease,” explains Muppidi. “Now that we have this model that can better represent aspects of human disease, we can try to understand what cues might affect the development of DLBCL,” he says. Potential next steps may include testing out combinations of therapies that can suppress the development of the disease.