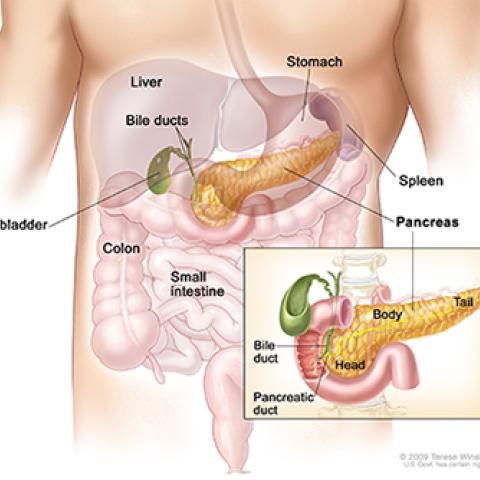
Anatomy of the pancreas.
Photo credit: NCI Visuals Online
Adults with pancreatic cancer that cannot be cured by surgery may be eligible to participate in a clinical trial at the NIH Clinical Center.
Udo Rudloff, M.D., Ph.D., Senior Investigator in the Pediatric Oncology Branch, is leading a study for people with advanced pancreatic cancer. Fewer than 10 percent of people with pancreatic cancer can have surgery, which offers the best chance of a cure. Radiation therapy is often used in locally advanced pancreatic cancer that cannot be removed in order to make surgery possible. Radiation shrinks the tumor so that a surgeon has a better chance of removing it entirely. For this study, participants will take two immunotherapy drugs (M7824 and M9241) either alone or with radiation. Participants who undergo stereotactic body radiation therapy (SBRT) will get it five days in a row during the first month of treatment. SBRT delivers extremely precise, very intense doses of radiation to cancer cells, which disrupts their DNA. M7824 and M9241 work in different ways to attack and kill cancer cells with damaged DNA.
Investigators want to see if these two drugs will work with SBRT to induce tumor shrinkage. Participants whose tumors shrink will go on to have surgery. For the initial phase of the study, where M7824 and M9241 are given alone, patients with stage IV disease that has spread to other organs may be eligible to participate.
Clinicaltrials.gov identifier: NCT04327986
NCI Protocol ID: NCI-20-C-0074
Official Title: A Phase I/II Study of the Immune Checkpoint Inhibitor M7824 and the Immunocytokine M9241 in Combination With Stereotactic Body Radiation Therapy (SBRT) in Adults With Advanced Pancreas Cancer
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.