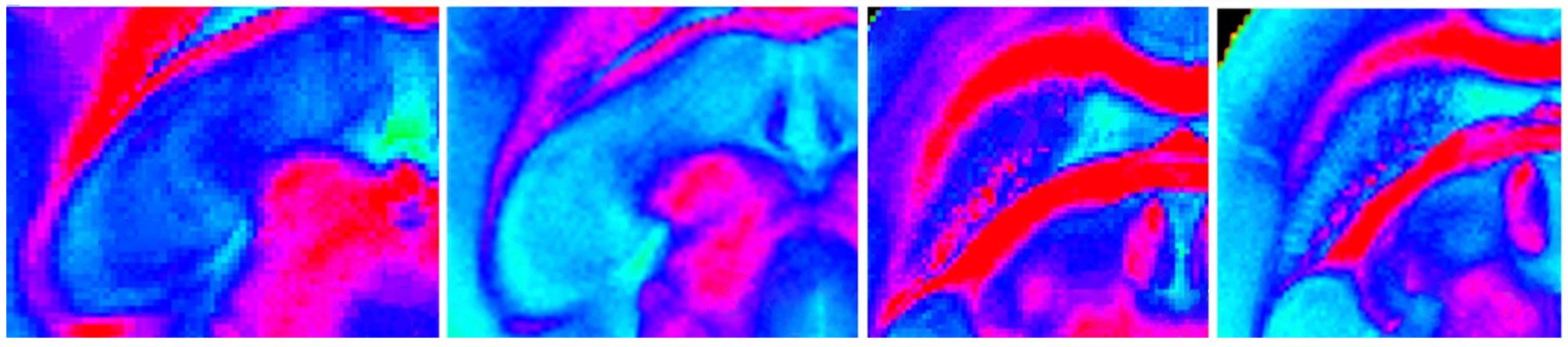
Preclinical experiments reveal that an alteration in the circadian PER2 gene may help prevent some of the negative side effects that brain tumor patients experience from radiation therapy.
Raleigh McElvery, Scientific Communications Editor
November 8, 2023
While cancer research often focuses on prolonging survival, ensuring patients feel good day-to-day is just as important. In fact, treatments that extend a person’s life often have side effects that diminish quality of life.
In a set of preclinical experiments published in Neuro-Oncology Advances, researchers in the lab of Terri Armstrong, Ph.D., at the NCI Center for Cancer Research’s Neuro-Oncology Branch (NOB) showed that a specific genetic alteration could help identify which brain tumor patients are at risk for certain adverse side effects. The results could advance personalized therapies and improve the treatment experience.
Cranial radiation therapy—high doses of radiation targeted at specific brain areas—is a standard treatment for people who are newly diagnosed with primary brain tumors. And yet, 90 percent of those undergoing cranial radiation experience excessive daytime sleepiness known as hypersomnolence. The severity and duration can be difficult to predict, but hypersomnolence often worsens during and immediately after radiation. In some cases, this daytime sleepiness can persist for months or remain unresolved. It can also be associated with anxiety, depression, and cognitive impairments. Some patients appear to be more prone to these side effects than others. For example, female brain tumor patients are nearly three times more likely to report fatigue-like symptoms than males.
Despite the prevalence of this cranial radiation-induced hypersomnolence, its biological basis remains unclear. To better understand who is (and who is not) at risk, researchers in the Armstrong Lab conducted a series of experiments in mice. Their results show that a mutation in a gene called PER2 prevents both daytime sleepiness and changes in mood post-treatment.
“By identifying the biological underpinnings leading to hypersomnia, we can target ways to prevent it from occurring—or lessen the severity in those at risk,” Dr. Armstrong says. “This has the potential to not only improve an individual’s life quality, but potentially improve treatment tolerance as well.”
Credit: Courtesy of the researchers
Back in 2017, Dr. Armstrong’s lab found that this particular PER2 mutation decreases the likelihood that brain tumor patients experience fatigue. PER2 is a type of gene called a clock gene that helps generate the circadian rhythm (the 24-hour internal clock that regulates the sleep/wake cycle). PER2 allows your body to “keep time” and maintain optimal patterns of daily functioning—for example, sleeping at night and remaining awake during the day. To further explore the role of genetics in symptom development, Dr. Armstrong’s team spent years developing a mouse model that replicates the cranial radiation-induced hypersomnolence that people experience.
In the new study, led by former NOB Postdoctoral Fellow Dorela Shuboni-Mulligan, Ph.D., and former NOB Postbaccalaureate Fellow Kendra Adegbesan, the researchers modified their existing model by inserting a piece of the human PER2 gene into the mouse genome. A subset of the mice had the PER2 mutation, while others did not. None of the mice had brain tumors.
The researchers monitored the animals’ general activity and sleep during the 10 days leading up to radiation and the 10 days afterward. Mice lacking the PER2 mutation experienced hypersomnolence post-radiation, indicated by a decrease in general activity and a tendency to sleep more. By contrast, the mice with the PER2 mutation did not experience those negative effects and were generally more active. In particular, female mice with the PER2 mutation showed reduced hypersomnolence over time. They also slept more immediately after radiation, which the researchers suspected aided their recovery.
To determine whether the mice also experienced the same mood and cognitive changes post-treatment as people with brain tumors, the researchers conducted several behavioral experiments. Roughly two months after radiation, they watched the mice complete tests measuring depression, anxiety, and working memory. The mice with the PER2 mutation displayed less anxiety-like behavior and were more active across all the behavioral tests.
“Our study shows that this PER2 mutation has a clear impact on symptom development after radiation,” says Dr. Shuboni-Mulligan, now an assistant professor of pathology and anatomy at Eastern Virginia Medical School. “This mutation seems to help protect against hypersomnolence—even in female mice who might be more susceptible.”
Study co-author Lino Tessarollo, Ph.D., headed the team that created the study’s mouse model. He says it’s the ideal animal model for studying the effects of this human genetic mutation on hypersomnolence post-treatment. Comparing the group of mice with the mutated human PER2 gene to those with the non-mutated gene allowed for “a perfectly controlled experiment,” he explains.
“At this stage, the study has genetically validated—beyond a clinical association—that this mutation plays a role in cranial radiation-induced hypersomnolence,” says Dr. Tessarollo, director of the Center for Cancer Research's Mouse Cancer Genetics Program. Future experiments, he explains, “will help us to develop a targeted pharmacological intervention for patients with this PER2 mutation who are undergoing brain radiation therapy.”
Although the researchers are still determining the biological causes of hypersomnolence, they suspect it could be due, in part, to the damaging effects of radiation. This treatment works by breaking the cancer cells’ DNA and killing them. But sometimes it can injury healthy tissue as well, including brain regions involved in regulating sleep and mood. The PER2 gene is known to regulate DNA repair, so perhaps it helps healthy cells recover from radiation damage by fixing their DNA.
In order to determine how their preclinical findings apply to patients, Dr. Armstrong’s team is running a clinical trial to assess daytime sleepiness and activity patterns in adults with brain tumors. Participants fill out questionnaires and receive a wearable device called a Fitbit to capture detailed data about their sleep and circadian rhythms.
Dr. Shuboni-Mulligan helped to start this clinical trial while she was at the NOB. “I loved getting the chance to interact with patients,” she says. “It’s been moving to meet people suffering from sleep problems and see how our research gives them hope.”
As the researchers continue to work toward improving patient quality of life, they are excited to watch in real-time as their bench-side research informs their bedside care—and vice versa.
Top image caption: In previous experiments, the researchers found that exposing mice to radiation damages brain tissue, which may affect cognitive function and worsen sleep symptoms. Credit: Courtesy of the researchers