Dr. Zhengping Zhuang combines clinical observations and preclinical models to understand how brain tumor cells multiply rapidly in oxygen-depleted environments during tumor development.
By Raleigh McElvery, Scientific Communications Editor
March 3, 2023
In 2012, Zhengping Zhuang, M.D., Ph.D., came across a curious clinical case. Four female patients between the ages of 20 and 30 were experiencing seemingly disparate ailments: neuroendocrine tumors and high red blood cell counts. Dr. Zhuang and his colleague Karel Pacak M.D., Ph.D., were initially puzzled by this phenomenon, until they pinpointed a unique genetic mutation that all four individuals shared—an alteration in genetic code affecting a protein known as hypoxia-inducible factor 2α (HIF2α). The World Health Organization has since named the group of symptoms arising from this mutation “Pacak-Zhuang syndrome.”
Now the head of the Cancer Stem Cell Biology Research Program at the NCI Center for Cancer Research’s Neuro-Oncology Branch (NOB), Dr. Zhuang is leveraging his knowledge of Pacak-Zhuang syndrome to develop more effective therapies for people with a different diagnosis: a very aggressive type of brain cancer known as glioblastoma.
Applying the Science of Pacak-Zhuang Syndrome to Brain Cancer
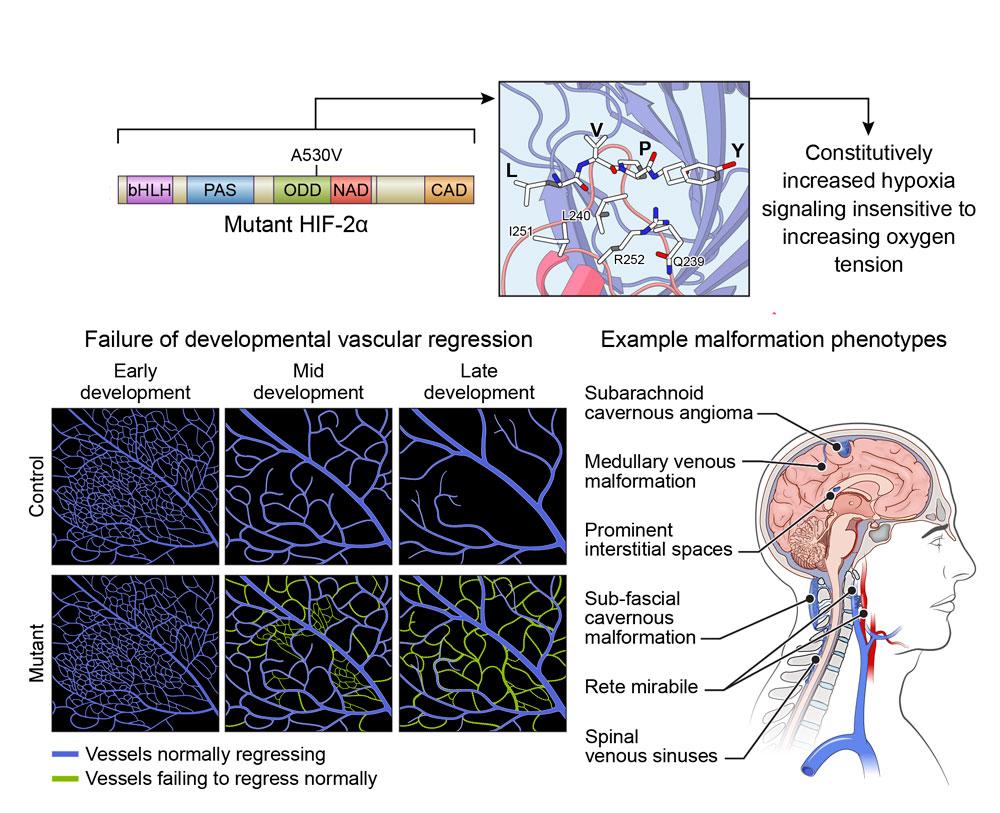
The protein HIF2α controls many important cellular processes, including how a cell adjusts to low-oxygen (“hypoxic”) environments. However, the mutation that causes Pacak-Zhuang syndrome prevents HIF2α from accurately sensing the oxygen levels around a cell. This activates a chain of molecular reactions known as the hypoxia signaling pathway, causing HIF2α to behave as though normal, healthy cells are starved of oxygen. As a result, the protein accumulates, leading to the phenomena that Dr. Zhuang first observed in 2012—as well as additional symptoms such as increased blood vessel growth.
At the time, Dr. Zhuang was a pathologist at the NIH’s Surgical Neurology Branch. He noticed many parallels between Pacak-Zhuang syndrome and the brain and spinal cord tumors he analyzed, including glioblastomas. Much like Pacak-Zhuang syndrome, these brain tumors are associated with an accumulation of HIF2α and other related HIF family proteins, as well as increased blood vessel growth.
In the early stages of tumor development, glioblastomas rely on active hypoxia signaling pathways to generate high HIF levels so the cells can multiply quickly—just like normal, non-cancerous stem cells do during development or regeneration. However, while normal cells eventually slow their growth once they’ve reached maturity, glioblastoma cells never stop rapidly dividing. As a result, they quickly outgrow the available oxygen supply and continue demanding high HIF levels to survive. Low oxygen environments are typically toxic to cells but—thanks to these HIF proteins—glioblastoma cells can thrive.
Glioblastomas are the most common malignant primary brain tumor, with over 12,000 new cases diagnosed each year in the United States. However, despite their prevalence, prognosis remains poor. Using his understanding of HIF proteins, Dr. Zhuang hopes to develop more effective therapies that target the hypoxia signaling pathway and improve outcomes for people with glioblastoma.
“Pacak-Zhuang syndrome provides a unique opportunity to study the role that HIF2α and other HIF proteins play in brain tumor development,” he says. “If we understand the molecular processes that allow cancer cells to survive in low oxygen environments, we can find therapeutic targets with the potential to help patients.”
Preclinical Innovations Inform Clinical Trials
In the five years since he arrived at the NOB, Dr. Zhuang has been developing a mouse model of Pacak-Zhuang syndrome. This has helped him verify the genetic underpinnings of the syndrome, while simultaneously delineating the role of HIF2α in glioblastoma development. In addition, he has also been working to identify potential therapeutic targets. For example, his preclinical research on a cancer drug called LB100 laid the foundation for a Phase 2 trial treating people with recurrent glioblastomas.
Most recently, Dr. Zhuang has focused on a type of immunotherapy known as chimeric antigen receptor T-cell therapy (CAR T-cell therapy). His team is engineering immune cells called CAR T cells that are designed to find and destroy the CAIX protein. CAIX is produced by glioblastoma cells under very hypoxic conditions as a result of high HIF protein levels. Although the researchers are still testing this therapeutic approach in their mouse models, they are currently preparing to launch a Phase 1 clinical trial to evaluate the treatment in people with glioblastoma.
In total, Dr. Zhuang holds five patents related to various scientific procedures and drug candidates. He also has 11 patents pending, including a cancer vaccine and a method that allows pathologists to view tissues samples in 3D.
“I enjoy innovation,” he explains. “I am an opportunist in the sense that I jump at the chance to invent approaches that could eventually improve treatment for cancer patients.”
Translating Discoveries from the Bench to Bedside and Back Again
As Dr. Zhuang investigates the role that HIF2α plays in Pacak-Zhuang syndrome and glioblastoma, he continues to reveal previously unknown facets of tumor development. At the NOB, he enjoys witnessing his laboratory discoveries become translated into clinical trials that aid people with brain tumors. He also relishes mentoring the trainees who join his lab—including former research fellow Chunzhang (Spring) Yang, Ph.D., who currently leads the NOB’s Molecular and Cell Biology Research Program.
“As a physician-scientist, I have a certain style of research,” Dr. Zhuang says. “I let observations from the clinic guide my work in the laboratory and vice versa.”
It’s this ethos that led him to discover Pacak-Zhuang syndrome over a decade ago, and—Dr. Zhuang hopes—will spur many more innovations to come.