Researchers discovered that a novel chemotherapy, PLX038, can penetrate the blood-brain-barrier to reach and kill tumor cells in glioblastoma mouse models. The lab work has already led to a clinical trial with hopes of better treatment outcomes for patients with difficult to treat brain and spine cancers.
By Brittany Cordeiro, Health Communications Specialist
September 10, 2024
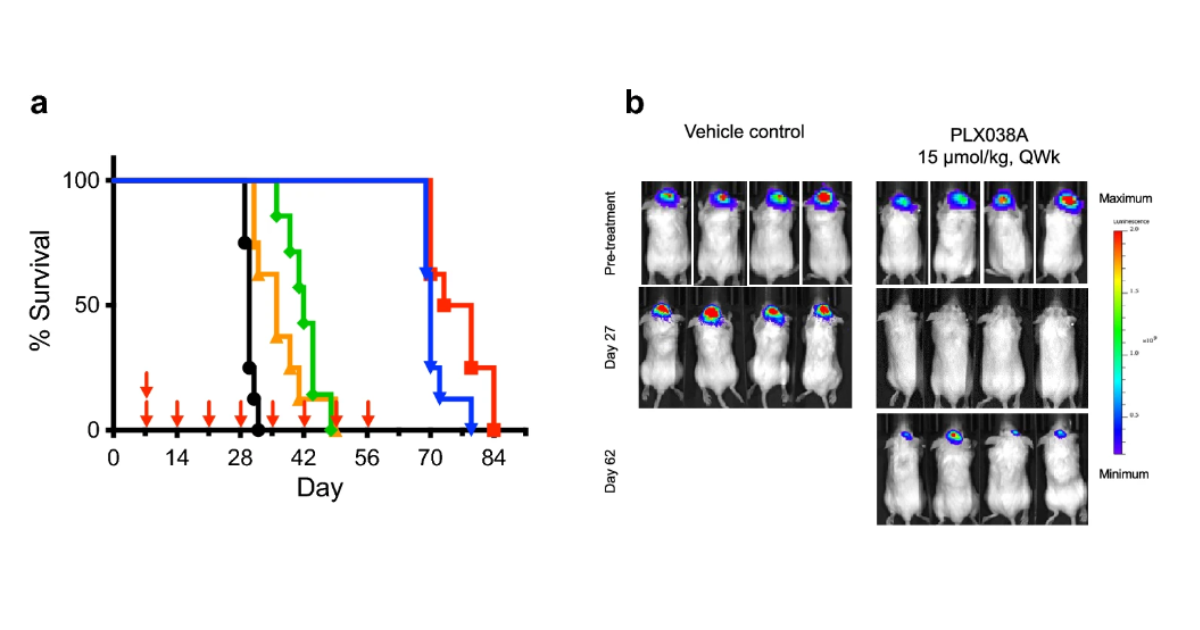
Researchers at the NCI Center for Cancer Research’s Neuro-Oncology Branch (NOB) have shown for the first time that a chemotherapy called PLX038 was highly effective at treating glioblastoma in preclinical models. The study published in Scientific Reports.
Glioblastoma is a fast-growing primary brain cancer that is difficult to treat. In part because most drug therapies cannot cross the blood-brain-barrier—a layer of cells that act as a shield to keep out harmful toxins, germs, and viruses from the brain. While this is good for brain health, for people with brain tumors, it can prevent tumor-killing drugs from reaching their target.
In the new study, lead author, Jinkyu Jung, Ph.D., a staff scientist in the NOB’s Precision Medicine Research Program, says the researchers showed that PLX038 was able to penetrate the blood-tumor-brain-barrier, accumulate in the tumor, and release its “cargo”—the potent molecule called SN-38 that damages tumor cells’ DNA.
“Our findings strongly support a novel tumor-penetrating mechanism for PLX038 in glioblastoma in vivo models,” says Dr. Jung. “This targeted approach can disrupt cancer cell proliferation more effectively than some conventional therapies, potentially leading to better treatment outcomes.”
How PLX038 Works to Increase Survival
PLX038 is a modified chemotherapy. “It works by targeting and modulating key signaling pathways crucial for glioblastoma cell proliferation and survival,” says Dr. Jung.
Specifically, PLX038 releases SN-38, which is a very potent inhibitor of the enzyme topoisomerase I (TOP1). The TOP1 enzyme is part of an elaborate mechanism that repairs damaged DNA inside cancer cells. SN-38 inhibits this enzyme, preventing DNA repair, and causing the cancer cells to die.
PLX038 is based on another FDA-approved chemotherapy called irinotecan, which is converted to SN-38 as its active ingredient. Irinotecan has proven to work well in other cancers such as colon and lung, and on brain cancer cells in a petri dish.
“SN-38 is very potent, but it only lasts perhaps 15 minutes in the blood and then it is gone,” says Mark Gilbert, M.D., scientist emeritus at NIH, and former NOB chief and head of the Precision Medicine Research Program. “If it takes any time for SN-38 to cross the blood-brain-barrier into the tumor, a lot of it's going to be gone before it ever gets there.”
The researchers decided to test PLX038 in glioblastoma because of its sustained release mechanism. This mechanism enables there to be good levels of SN-38 in the blood for hours rather than minutes. This means that SN-38 could survive longer in the bloodstream to reach its target. Prior research discovered that PLX038 works by stabilizing SN-38 on a polyethylene glycol molecule or basically, a glob of fat. Over time, about two to three weeks, SN-38 leeches out.
“Rather than there being a surge of SN-38 that gets degraded, there's constant concentration,” explains Dr. Gilbert, an author on the study. “Imagine PLX038 as a bar of soap as it dissolves. The SN-38s are the soapy pieces coming off.”
The study revealed this novel mechanism of action, and the result was an increase in survival of mice with glioblastoma. “For such difficult to treat brain cancers, PLX038 could provide new therapeutic options, potentially offering improved efficacy over existing treatments,” says Dr. Jung.
PLX038 Offers Hope in New Clinical Trial
The study results supported the development of a new phase 1/2 clinical trial using PLX038 and led by NCI-CONNECT (Comprehensive Oncology Network Evaluating Rare Central Nervous System (CNS) Tumors). The trial tests whether PLX038 can treat adults with rare primary CNS cancers with extra copies of genes called MYC and MYCN (known as MYC and MYCN amplified tumors). These tumors may be especially sensitive because they rely heavily on TOP1 to fix their DNA, shared Marta Penas-Prado, M.D., study principal investigator and NOB senior clinician. The clinical trial will be the first to test this chemotherapy in people with CNS tumors.
“The next steps are to refine the molecular profile of the tumors that are responsive in the clinical trial,” says Dr. Gilbert, “Then, you are only treating patients with a high likelihood of response.”
The study and subsequent clinical trial are a perfect “proof of principal” for precision medicine, says Dr. Gilbert. “You can find predictive biomarkers if you do the appropriate preclinical studies, and then you do validation in a clinical trial. This will help determine which patients are most likely to respond to treatment.”