Molecular Pathology Test Directory Laboratory of Pathology
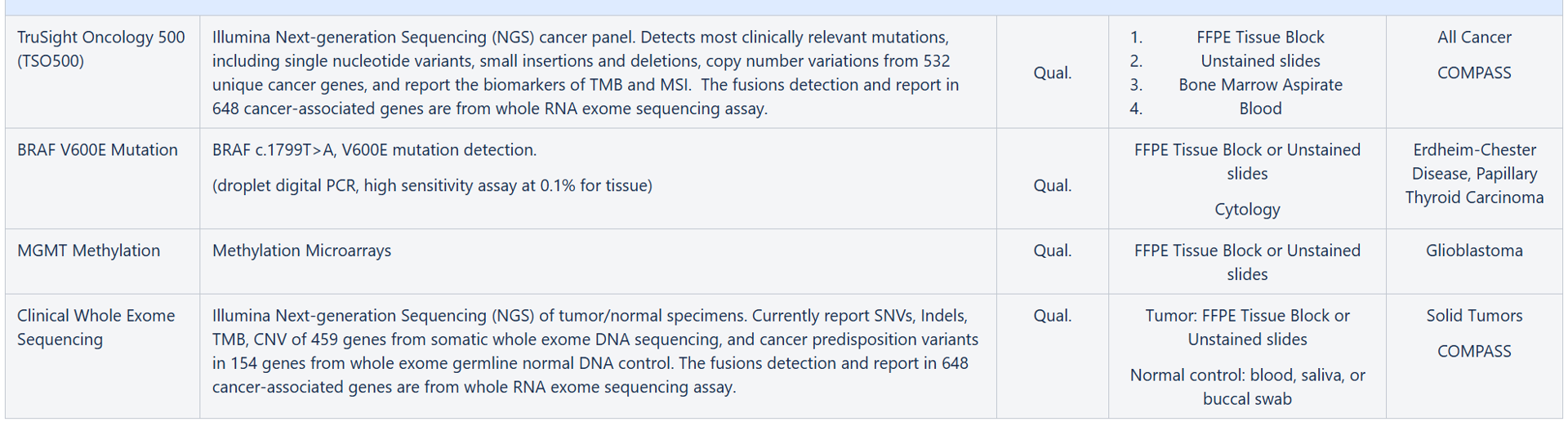
TSO500 DNA Assay Gene Panel (532 Genes)
RNA Exome Fusion Panel (648 Genes)
Genes for reporting cancer predisposition variants from Clinical Whole Exome Sequencing (154 Genes)
A molecular pathology CRIS order is required prior to submission of a specimen for molecular pathology testing. The CRIS order is listed under the heading of Anatomic Pathology, sub-heading, Molecular Pathology. The molecular pathology order is driven by specimen sources which are divided into 5 types.
When entering CRIS order for a requested physician, please enter REQUESTED BY physician’s name. This will assure that the requested physician’s name appears correctly on clinical report.
1. Blood Specimen
- In CRIS: Order Entry / Anatomic Pathology / Molecular Pathology, Blood.
- Enter a collection date which accurately reflects the date of specimen collection.
- Enter in Brief Clinical History to communicate with molecular pathologists.
- From “Test Requested”, select Hematopathology and check one or more tests. Or from “Test Requested”, select Exome Germline Variant Control.
- Or from “Test Requested”, select COMPASS and check TruSight Oncology 500 (TSO500)
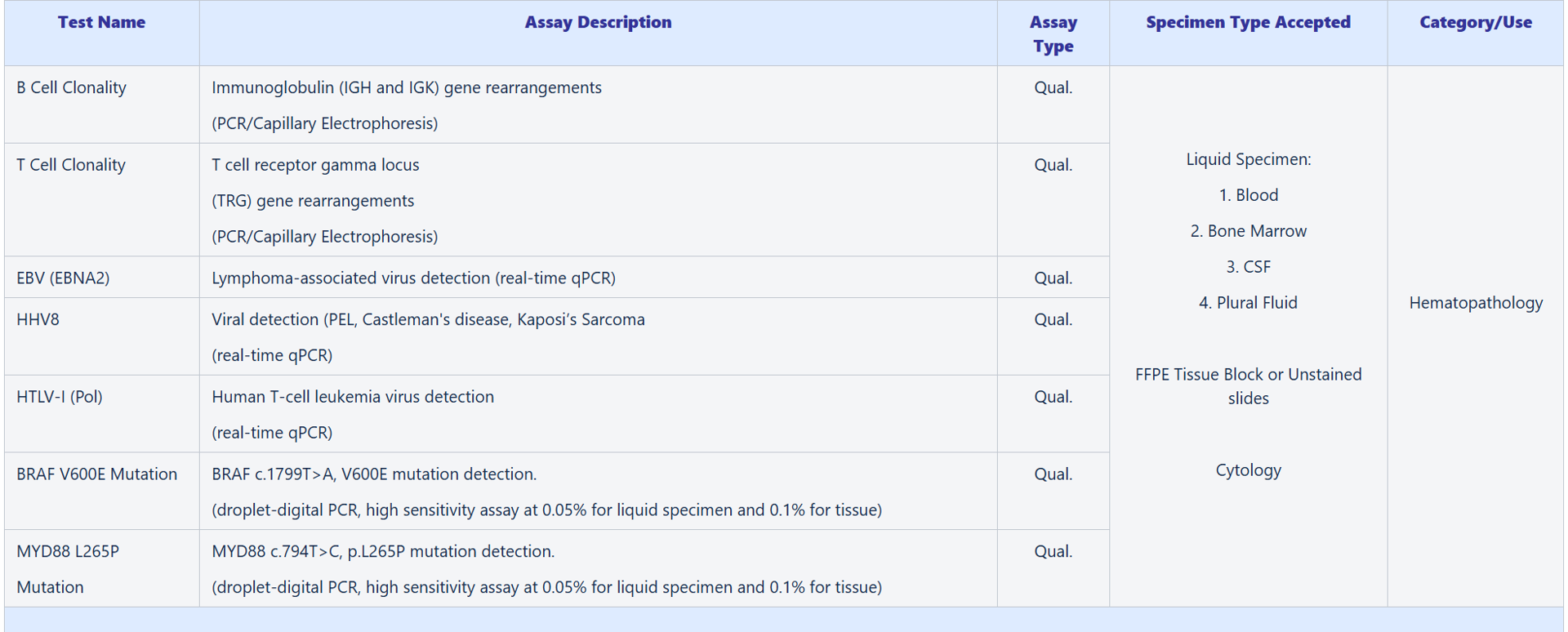
Available Tests – Hematopathology:
- B Cell Clonality
- T Cell Clonality
- EBV (EBNA2)
- HHV8
- HTLV-1
- BRAF V600E Mutation
- MYD88 L256P Mutation
Available Tests – COMPASS:
- Exome Germline Variant Control (for Whole Exome DNA and RNA Sequencing)
- TruSight Oncology 500 (TSO500)
Also see liquid specimen collection and handling instructions (below).
2. Bone Marrow Aspirate Specimen
- In CRIS: Order Entry / Anatomic Pathology / Molecular Pathology, Bone Marrow.
- Enter a collection date which accurately reflects the date of specimen collection.
- Enter in Brief Clinical History to communicate with molecular pathologists.
- From “Test Requested”, select Hematopathology
- from “Test Requested”, select COMPASS and check TruSight Oncology (TSO500)
Available Tests – Hematopathology:
- B Cell Clonality
- T Cell Clonality
- EBV (EBNA2)
- HHV8
- HTLV-1
- BRAF V600E Mutation
- MYD88 L256P Mutation
Available Tests – COMPASS:
- TruSight Oncology 500 (TSO500)
Also see liquid specimen collection and handling instructions (below).
3. CSF or Pleural Fluid Specimen
- In CRIS: Order Entry / Anatomic Pathology / Molecular Pathology, Body Fluid.
- Enter a collection date which accurately reflects the date of specimen collection.
- Enter in Brief Clinical History to communicate with molecular pathologists.
- From “Specimen Source”, select type of body fluid.
- From “Test Requested”, select Hematopathology and check one or more tests.
Available Tests – Hematopathology:
- B Cell Clonality
- T Cell Clonality
- EBV (EBNA2)
- HHV8
- HTLV-1
- BRAF V600E Mutation
- MYD88 L256P Mutation
Also see liquid specimen collection and handling instructions (below).
4. Tissue Specimen
- In CRIS: Order Entry / Anatomic Pathology / Molecular Pathology, Tissue.
- Enter a collection date which accurately reflects the date of specimen collection.
- Enter in Brief Clinical History to communicate with molecular pathologists.
- From “Test Requested”, select one of 3 test categories (Hematopathology, Single Mutation, COMPASS) to see and select one or more tests.
- If COMPASS Whole Exome DNA and RNA Sequencing is selected, you must obtain a written consent from the patient AND put in a separate CRIS order of Exome Germline Variant Control with two options of control materials #1: Molecular Pathology, Blood or #2: Molecular Pathology, Outside Material Other (Type-In) Saliva. Please check consenting and ordering SOP# ADGC-5 .
Available Tests – Hematopathology:
- B Cell Clonality
- T Cell Clonality
- EBV (EBNA2)
- HHV8
- HTLV-1
- BRAF V600E Mutation
- MYD88 L256P Mutation
Available Tests – Single Mutation:
- BRAF V600E Mutation
- MYD88 L256P Mutation
Available Tests – COMPASS:
- TruSight Oncology 500 (TSO500)
- Whole Exome DNA and RNA Sequencing
Also see FFPE tumor tissue requirement and submission instructions (below).
5. Outside Material (only for blood or bone marrow received in your clinic from outside of the NIH Clinical Center or saliva from patient)
- In CRIS: Order Entry / Anatomic Pathology / Molecular Pathology, Outside Material.
- Enter a collection date which accurately reflects the date of specimen collection.
- Enter in Brief Clinical History to communicate with the molecular pathologists.
- From “Specimen Source”, select Blood, or Bone Marrow, or Other (Type-In).
- From “Test Requested”, select one test category from dropdown list.
- Check the test(s) to order.
- Label the specimen tube with patient name and NIH MRN. Bring the specimen to the room 3S247.
Available Tests – Hematopathology:
- B Cell Clonality
- T Cell Clonality
- EBV (EBNA2)
- HHV8
- HTLV-1
- BRAF V600E Mutation
- MYD88 L256P Mutation
Available Tests – COMPASS:
- Exome Germline Variant Control (for Whole Exome DNA and RNA Sequencing)
Also see liquid specimen collection and handling instructions (below).
Note: Specimens delivered to Room 3S247 in Building 10 will be accepted from 7:30 AM to 4:30 PM Monday to Friday.
When submitting patient samples to the Molecular Pathology, the requesting physician or nurse must enter a CRIS order prior to submission of the specimens.
Blood:
- 3 ml of whole blood is collected in Sodium Citrate tube (one tube only).
- The tubes are wrapped in plastic bags (at room temperature) and sent immediately to room 3S247.
Bone Marrow Aspirates:
- Draw 2 ml in plain syringe with no anticoagulant. DO NOT USE HEPARINIZED SYRING
- IMMEDIATELY place sample into the EDTA tube (lavender top) and mix gently to prevent clotting.
CSF:
2-3 ml. Samples are placed in a CSF collection tube, wrapped in a plastic bag and then placed in a second bag or cup with wet ice. At no time should the ice and the tube have direct contact.
Pleural Fluid:
2-15 ml. Samples are placed in a capped conical tube, wrapped in a plastic bag and then placed in a second bag or cup with wet ice. At no time should the ice and the tube have direct contact.
Saliva:
Use saliva collection from Orange DNA-OG500 and follow the instruction in the kit.
Buccal Swab:
Use buccal swab collection kit – ORACOLLECT-Dx OCD-100 and following the instruction in the kit.
Specimen Collection Table
1. CRIS orders for both Molecular and Surgical Pathology
All FFPE tissues to be performed for molecular pathology testing should be submitted routinely through Surgical Pathology. It is essential to submit a concurrent Surgical Pathology CRIS if the tissue has not been reviewed by the Laboratory of Pathology.
For all patient orders to have results available in the CRIS system, it is necessary to enter the order into the CRIS system.
2. Specimen and Handling Requirements
1. A new tissue specimen is being submitted from a procedure performed at the NIH Clinical Center or outside. In this case, two separate CRIS orders are required - one for surgical pathology or cytopathology to request routine pathology services, and a second CRIS order for the molecular pathology test needed. Please also indicate in the special instructions box on the surgical pathology or cytopathology CRIS order that molecular testing is being requested on the sample and, if appropriate, that tissue should be prioritized for molecular testing.
2. For cases that have been already been signed out by LP, the tissue specimens are retained in LP. A single molecular pathology CRIS order is required to trigger the molecular testing. Please indicate the LP surgical case number in the Special Instructions, if known (this number can be obtained from the LP pathology report).
3. All specimens from in-house surgery and outside materials (surgical specimens, tissue blocks, stained and unstained slides, and outside reports) are to be brought directly to General Surgical Pathology office - 10/Room 2S262 (open 7:30 AM to 4:00 PM). The molecular diagnostics laboratory cannot accession cases.
4. Paraffin-embedded tissue specimens from outside must be formalin fixed (non-decalcified) and less than 10 years old. You may submit either a tissue block or a minimum of 10 unstained slides containing >20% tumor for surgical pathology review. Keep in mind that copy number alteration assessment will be compromised below 50% tumor content. DNA tests from 5-10 years old tissue have a significantly higher failure rate. RNA testing will not be performed for tissue more than 5 years old.
General Information
Building 10, Room 3S249
LP Molecular Pathology Main Number: 301-480-8080
LP Molecular Pathology Fax Number: 301-480-8926
Contacts:
Dr. Liqiang Xi, Staff Scientist
(301) 480-8933
To learn more, visit Dr. Xi's CCR Web site.
Winnifred Navarro, Medical Technologist (ASCP)
(301) 480-8928
Tina Pham, Medical Technologist (ASCP)
(301) 480-8929
Trinh Hoc-Tran Pham, Medical Technologist
(301) 480-8932
Jeffrey Hanson, Medical Technologist
(240) 760-7174
Lucia Chinchilla, Medical Technologist
(240) 858-3547
Thanhlong (Gilbert) Tran, Medical Technologist
(240) 858-7325
Michael Moretto, Medical Technologist
(240) 858-3629
Mailing Address:
Molecular Diagnostics Lab
Laboratory of Pathology, CCR
National Cancer Institute
10 Center Drive
Building 10, Room 3S249
Bethesda, MD 20892
Regular Working Hours:
9:00 a.m. to 6:00 p.m. (Monday - Friday, excluding federal holidays)