In Vivo Capabilities
- GEM engineering
- Characterization and validation of GEM models
- GEM model re-tooling/refinement
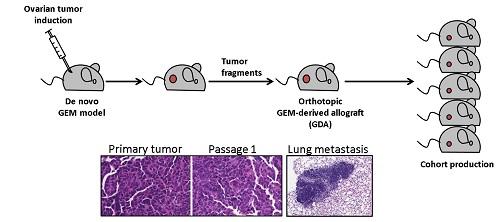
- Adaptation of GEM models to syngeneic allograft models
- Surgical techniques for generation of murine orthotopic transplant model cohorts
- Pharmacokinetics and biodistribution of therapeutics in animal models
- Pharmacodynamics: evaluation of drug effect on intended target in tumor tissue
- Evaluation of therapeutic candidates in efficacy studies
- Cancer prevention studies in GEM models
- Imaging modalities (MRI, PET/CT, ultrasound, and bioluminescence)
- Accurate real-time tumor volume measurements based on live imaging
- Biomarkers/molecular signatures of treatment response:
- Immunohistochemical stains
- Transcriptome
- Metabolome
- Generation of novel PDX models from patient tissue
In Vitro Capabilities
- ES cell derivation
- Primary tumor cell culture establishment
- iPSC technologies
- Cell viability assays
- CRISPR and recombineering allele construction
- Histopathological assessment
- Quantitative molecular pathology
- Evaluation of drug target inhibition
- Establishment of primary tumor cell culture from GEM, GDA or PDX models
- Evaluation of drug potency in primary tumor cells/specific target validation assays