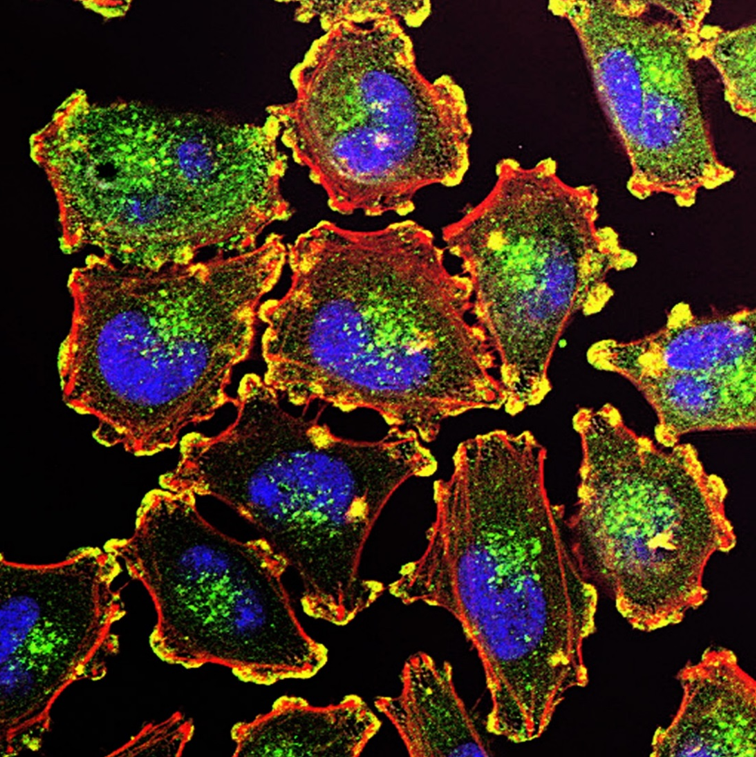
The ability of cancer cells to move and spread depends on actin-rich core structures such as the podosomes (yellow) shown here in melanoma cells. Cell nuclei (blue), actin (red), and an actin regulator (green) are also shown.
Credit: Julio C. Valencia, NCI, NIH
Cell-based therapies have long been a critical part of modern medicine. This includes use in blood transfusions, skin grafts, as well as transplantation of organs and bone-marrow. The potential therapeutic properties of ‘living cells as specific drugs’ in the treatment of cancer has more recently become clear. Recent advances at the NCI’s Center for Cancer Research (CCR) have shown that:
- Naturally-occurring T cells can trigger objective responses in patients with melanoma, colon cancer, breast cancer, cholangiocarcinoma and others.
- Gene-engineered T cells can cause regressions in patients with leukemia, lymphoma, melanoma and sarcoma.
An important goal is to adapt this approach for successful treatment of a variety of cancer types, including commonly occurring epithelial cancers such as carcinoma of the breast, lung, prostate and colon. This would bring this new and promising treatment to hundreds of thousands more patients each year. The CCR Center for Cell-based Therapy (CCT) was formed to accelerate this process.
The mission of the Center for Cell-based Therapy is to facilitate the discovery and development of cellular immunotherapies for patients with cancer
The CCR has long been a leader in developing cell-based treatments for cancer. The CCT aims to:
- Build on the CCR’s existing strengths in cell-based therapy
- Fortify natural synergies between existing collaborators and bring together NIH-wide expertise in immunology, gene therapy, gene editing and regenerative medicine.
- Create outstanding infrastructure to more rapidly advance cell-based immunotherapies
Dr. Steven A. Rosenberg speaking with a patient at the NIH Clinical Center.
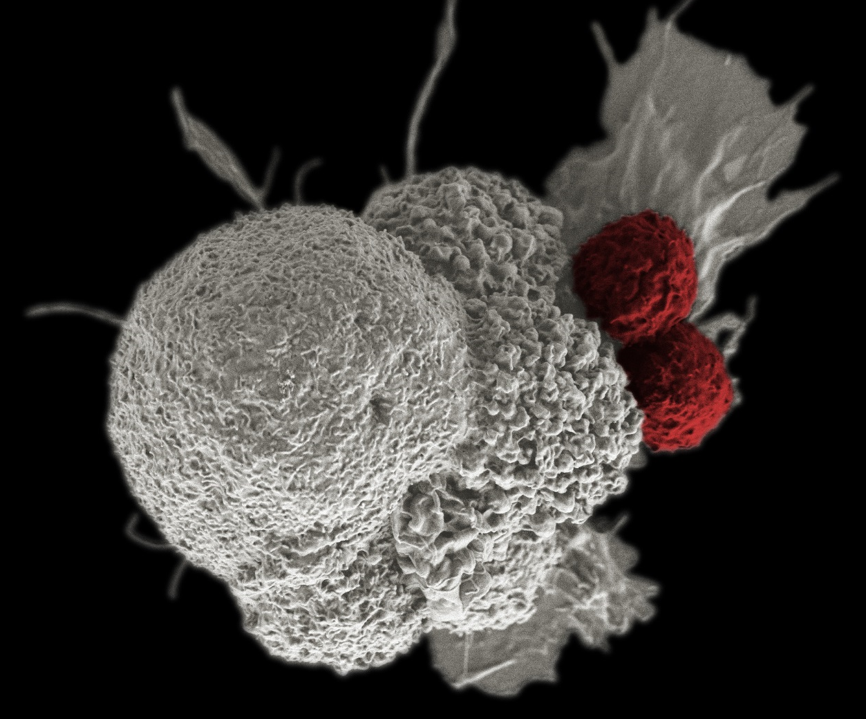
A pseudo-colored scanning electron micrograph of cancer cells (white) being attacked by two cytotoxic T cells (red).
Credit: Rita Elena Serda, Duncan Comprehensive Cancer Center at Baylor College of Medicine.
Specifically, the CCT seeks to:
- Build a Solid Foundation: By developing the principles of cell based therapies through strong basic research.
- Accelerate Translational Work: By enhancing the ability of basic research laboratories to translate their work into preclinical and clinical studies
- Facilitate Innovation: By taking early stage research and bringing it to the clinic.
- Teach and Train: By hosting visiting investigators and holding educational symposia.
- Share and Provide Access to Technology: By creating robust technology transfer to non-for-profit and to commercial entities.
- Create a Vision for the Future: By exploring new directions for the use of ‘cells as drugs.’
The CCT is part of the CCR, which is the largest division in the NCI intramural research program. Located on the Bethesda campus of the NIH, this is an ideal site for leading the emerging field of cell-based therapies. Advantages include:
- Broad expertise in the basic science of cell-based techniques.
- A world-class community of immunologists in the CCR, NCI and NIH with expertise spanning basic, translational and clinical research
- Access to the NIH Clinical Center, the Nation’s largest research hospital which is staffed with experienced teams of experimental clinicians.
- Unique patient populations for whom existing therapies have been insufficient.
- Established infrastructure for interacting with regulatory agencies in the absence of financial conflicts-of-interest.
- New efforts to increase the capacity for pre-clinical and clinical production of human cells. This includes a large GMP-quality cell production laboratory with cell sorting, as well as the capacity for GMP vector production and new space for completing at or near scale experimental pre-clinical work (eg CRISPR, iPSC).
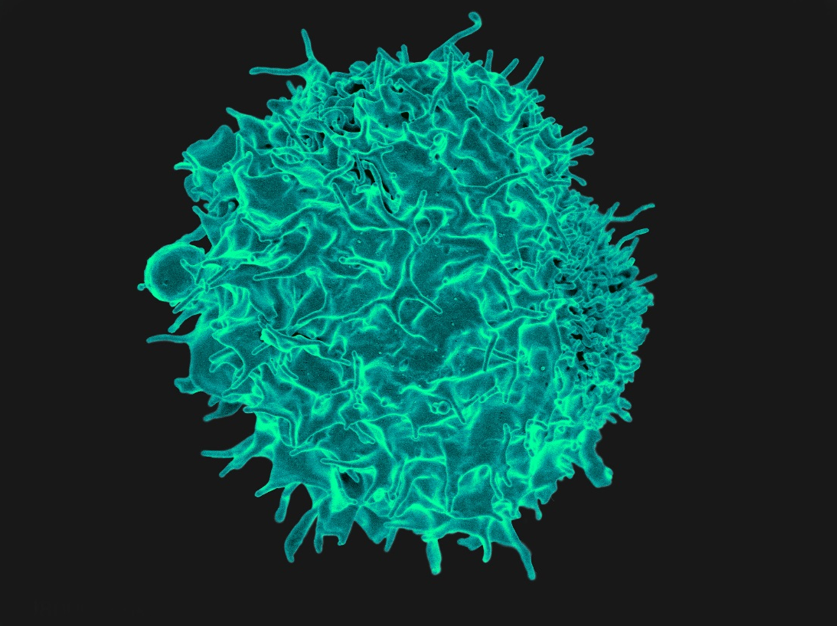
Scanning electron micrograph of a T lymphocyte, such as those used at the CCR to kill tumors in an approach known as adoptive T-cell immunotherapy.
Credit: NIAID, NIH
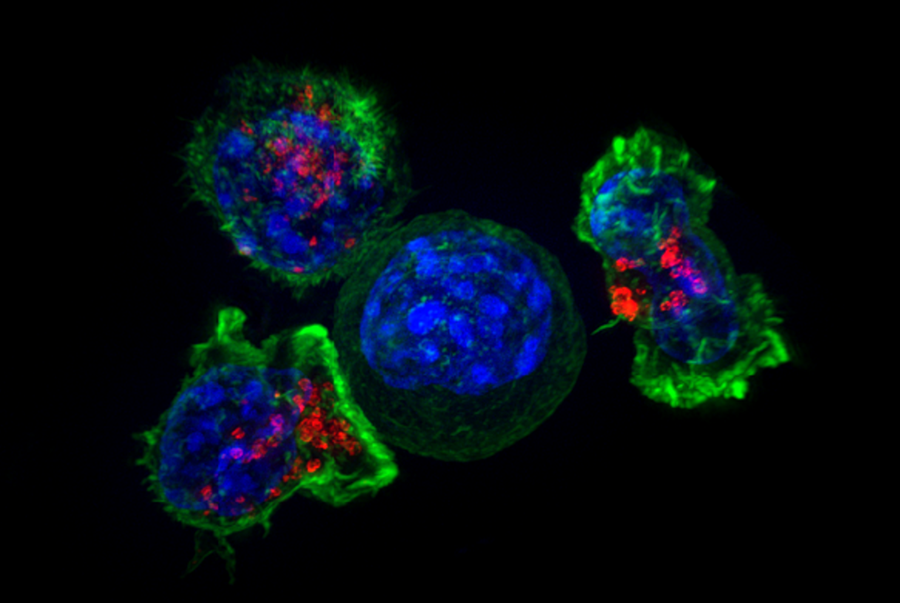
T cells surround and attack a cancer cell. CAR-T cells, first developed at the CCR, are made by inserting receptors into T cells and have been used to successfully treat some blood cancers.
Credit: Alex Ritter, Jennifer Lippincott Schwartz and Gillian Griffiths, NIH
In addition to maintaining long-standing collaborations, the CCT will capitalize on the considerable strength of the immunology community in the CCR, the NCI and the NIH through interaction with groups such as the: