
Yoshimi Endo Greer, M.D., Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building.10, Room4B50
- Bethesda, MD 20892-4256
- 240-858-3275
- greery@mail.nih.gov
RESEARCH SUMMARY
Yoshimi E. Greer, M.D., Ph.D., is an experienced scientist in cell biology, molecular biology, biochemistry and oncology. Her current research focus is cancer metabolism in breast cancers.
Areas of Expertise

Yoshimi Endo Greer, M.D., Ph.D.
Publications
Biography

Yoshimi Endo Greer, M.D., Ph.D.
Dr. Yoshimi E. Greer obtained her M.D. and Ph.D. in Tohoku University School of Medicine, Japan. Dr. Greer joined NIDDK/NIH in 1998 as a visiting post-doctoral fellow. She then joined NCI in 2001, and studied signal transduction in cancer. In 2004, she was appointed as a Research Instructor at the Lombardi Comprehensive Cancer Center, Georgetown University Medical School in Washington D.C. In 2006, she returned to NCI as a Research Fellow. In 2009, she was appointed as a Staff Scientist in the Laboratory of Cellular and Molecular Biology (LCMB), CCR. In 2014, she joined Dr. Stan Lipkowitz’s lab to conduct basic and translational research in breast cancer. In 2021, Dr. Greer is appointed as an Associate Scientist in the Women's Malignancies Branch, CCR.
Her current research focuses are 1) to develop breast cancer treatment strategies utilizing TRAIL/Death Receptor, 2) to investigate the role of mitochondria in cancer as a novel therapeutic target.
Covers
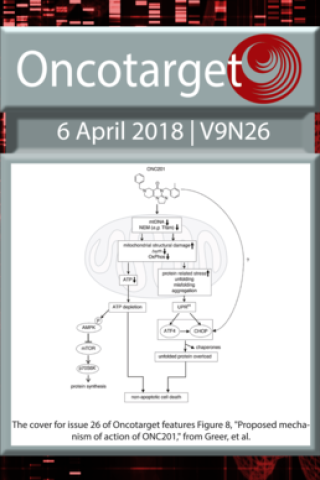
ONC201 kills breast cancer cells in vitro by targeting mitochondria
About the Cover
The cover for issue 26 of Oncotarget features Figure 8, "Proposed mechanism of action of ONC201" from Greer, et al.
Abstract
We report a novel mechanism of action of ONC201 as a mitochondria-targeting drug in cancer cells. ONC201 was originally identified as a small molecule that induces transcription of TNF-related apoptosis-inducing ligand (TRAIL) and subsequently kills cancer cells by activating TRAIL death receptors. In this study, we examined ONC201 toxicity on multiple human breast and endometrial cancer cell lines. ONC201 attenuated cell viability in all cancer cell lines tested. Unexpectedly, ONC201 toxicity was not dependent on either TRAIL receptors nor caspases. Time-lapse live cell imaging revealed that ONC201 induces cell membrane ballooning followed by rupture, distinct from the morphology of cells undergoing apoptosis. Further investigation found that ONC201 induces phosphorylation of AMP-dependent kinase and ATP loss. Cytotoxicity and ATP depletion were significantly enhanced in the absence of glucose, suggesting that ONC201 targets mitochondrial respiration. Further analysis indicated that ONC201 indirectly inhibits mitochondrial respiration. Confocal and electron microscopic analysis demonstrated that ONC201 triggers mitochondrial structural damage and functional impairment. Moreover, ONC201 decreased mitochondrial DNA (mtDNA). RNAseq analysis revealed that ONC201 suppresses expression of multiple mtDNA-encoded genes and nuclear-encoded mitochondrial genes involved in oxidative phosphorylation and other mitochondrial functions. Importantly, fumarate hydratase deficient cancer cells and multiple cancer cell lines with reduced amounts of mtDNA were resistant to ONC201. These results indicate that cells not dependent on mitochondrial respiration are ONC201-resistant. Our data demonstrate that ONC201 kills cancer cells by disrupting mitochondrial function and further suggests that cancer cells that are dependent on glycolysis will be resistant to ONC201.
ONC201 kills breast cancer cells in vitro by targeting mitochondria. Greer YE, Porat-Shliom N, Nagashima K, Stuelten C, Crooks D, Koparde VN, Gilbert SF, Islam C, Ubaldini A, Ji Y, Gattinoni L, Soheilian F, Wang X, Hafner M, Shetty J, Tran B, Jailwala P, Cam M, Lang M, Voeller D, Reinhold WC, Rajapakse V, Pommier Y, Weigert R, Linehan WM, and Lipkowitz S. Oncotarget 9(26):18454-18479, 2018.
About Oncotarget
Oncotarget is a weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology. To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with @Oncotarget
Oncotarget is published by Impact Journals, LLC. Please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
18009220957x105
MEDIA@ONCOTARGET.COM
Centrosomal CK1delta Promotes Neurite Outgrowth
Previously we determined that Dishevelled-2/3 (Dvl) mediate Wnt-3a–dependent neurite outgrowth in Ewing sarcoma family tumor cells. Here we report that neurite extension was associated with Dvl phosphorylation and that both were inhibited by the casein kinase 1 (CK1) δ/ε inhibitor IC261. Small interfering RNAs targeting either CK1δ or CK1ε decreased Dvl phosphorylation, but only knockdown of CK1δ blocked neurite outgrowth. CK1δ but not CK1ε was detected at the centrosome, an organelle associated with neurite formation. Deletion analysis mapped the centrosomal localization signal (CLS) of CK1δ to its C-terminal domain. A fusion protein containing the CLS and EGFP displaced full-length CK1δ from the centrosome and inhibited Wnt-3a–dependent neurite outgrowth. In contrast to wild-type CK1ε, a chimera comprised of the kinase domain of CK1ε and the CLS of CK1δ localized to the centrosome and rescued Wnt-3a–dependent neurite outgrowth suppressed by CK1δ knockdown. These results provide strong evidence that the centrosomal localization of CK1δ is required for Wnt-3a–dependent neuritogenesis.
DOI: 10.1083/jcb.201011111