Ira Pastan, M.D.
- Center for Cancer Research
- National Cancer Institute
- Building 37, Room 5106
- Bethesda, MD 20892
- 240-760-6470
- pastani@mail.nih.gov
RESEARCH SUMMARY
Dr. Pastan’s contributions to medical research include:
- The discovery with R. Perlman of a new gene regulatory mechanism whereby many quiescent genes are activated by cyclic AMP and its DNA-binding partner CRP.
- Receptor studies which provided the first evidence that peptide hormones bind to receptors on the surface cells, and the use of fluorescence methods to visualize the binding and internalization of insulin and other ligands by living cells, to measure their diffusion rates on the membrane before clustering and internalization, and to demonstrate that different ligands, including viruses, enter cells together in the same vesicles.
- Research on the EGF receptor, which showed that the EGF-R (onco)gene is amplified and over-expressed in many human cancers, the cloning and sequencing of the EGF-R cDNA and with Doug Lowy, the demonstration that over-expression of EGF-R in the presence of EGF can transform normal cells into cancer cells. These studies provided essential evidence that antibodies able to inactivate the EGF receptor could be useful in treating many cancers.
- Studies with M. Gottesman on the biochemical basis of multi-drug resistance in cancer therapy, which involved the isolation of the first human multi-drug resistance gene (MDR1) gene and the demonstration that MDR1 encodes a multi-drug transporter that is highly expressed in many human cancers.
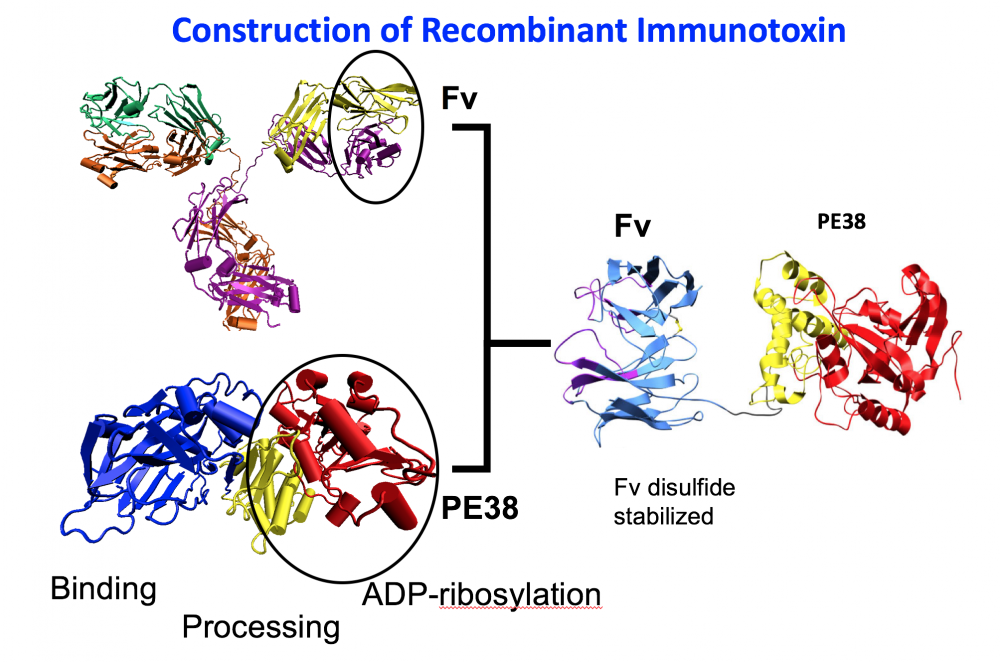
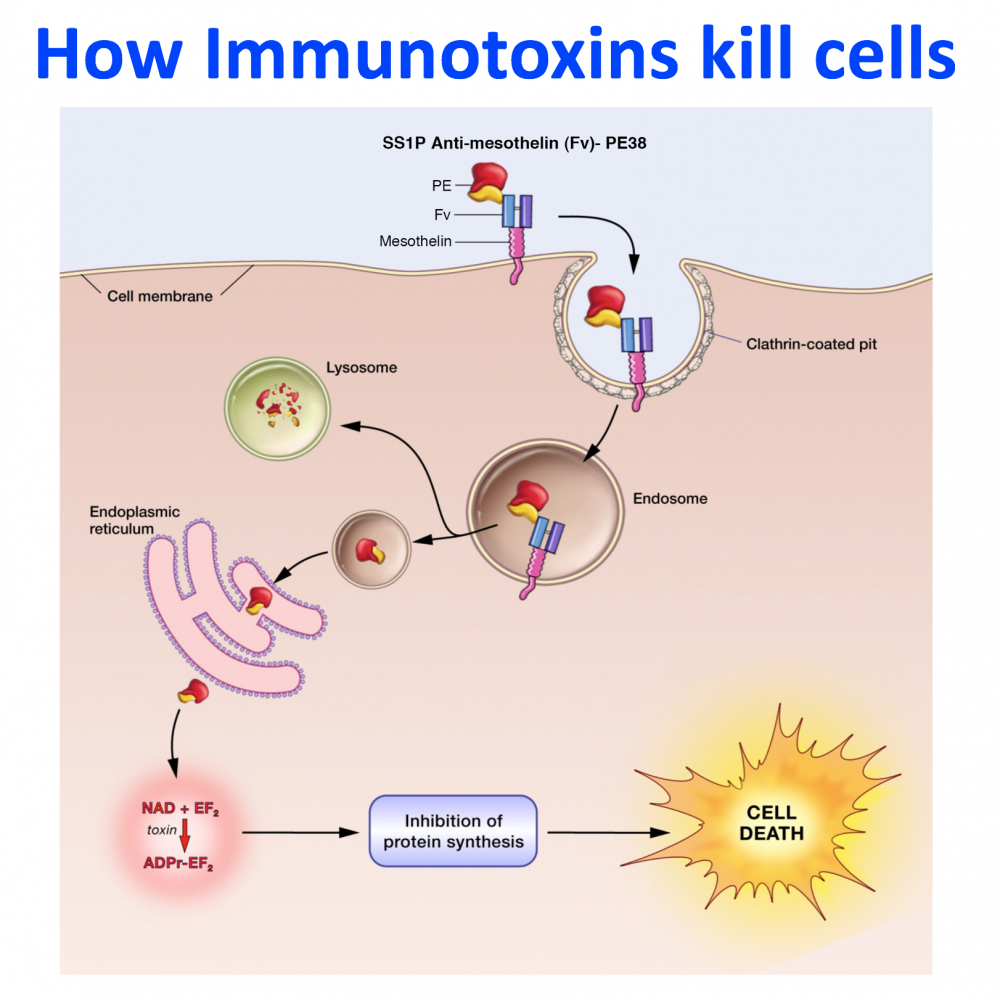
- The development of recombinant immunotoxins for the treatment of cancer.. Working with R. Kreitman and D. FitzGerald, I used protein engineering to produce novel chimeric proteins composed of an Fv that targets a protein on the surface of a cancer cell and a portion of a powerful protein toxin. One of these Lumoxiti has been approved by the FDA for the treatment of leukemia.
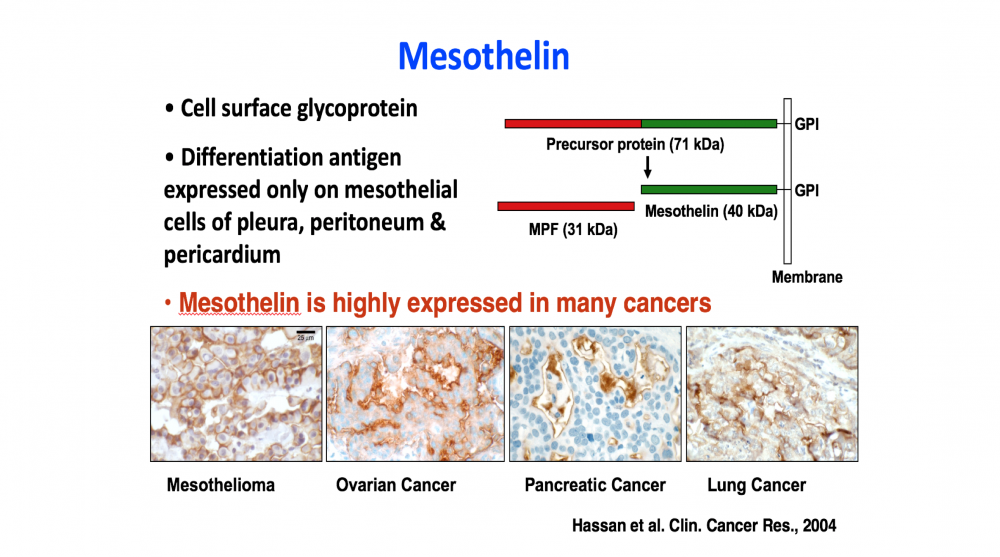
- The discovery of mesothelin (with M.C. Willingham) and the demonstration that it is an excellent target for antibody-based cancer therapies, because of its high expression on many cancers and lack of expression on essential normal organs.
Areas of Expertise
Research
Mesothelin as a Target for Cancer Therapy by CAR-T cells and Bispecific Antibodies
To improve the therapy of cancer new approaches and drugs with unique mechanisms of action are needed. Mesothelin is a popular target on solid tumors. It was discovered by Ira Pastan and Mark Willingham in LMB, NCI. It is a lineage restricted cell surface protein that is highly expressed by many cancers, and only a few non-essential tissues. The specificity of mesothelin expression makes it a popular target for antibody-based therapies, but shed mesothelin has been found to be a major barrier to these therapies. To overcome the shedding problem, Pastan and collaborators developed Mab 15B6 that binds to a juxta-membrane region of mesothelin and does not bind to shed mesothelin. Mab 15B6 was used to make CAR T cells and bispecific (T cell engagers that have high anti-tumor activity in mice. An improved CAR-T cell variant is being prepared for clinical trials in human with mesothelin expressing cancers.
Publications
- Bibliography Link
- View Dr. Pastan's PubMed Summary.
Biography

Ira Pastan, M.D.
Dr. Ira Pastan was educated at the Boston Public Latin School, Tufts College, and Tufts Medical School. He did his residency at the Yale School of Medicine (1957-1959) and came to NIH in 1959. In 1970, he founded the Laboratory of Molecular Biology in the NCI.
Dr. Pastan retired from CCR as Chief Emeritus of the Laboratory of Molecular Biology in March 2025.
Major honors
Van Meter Prize, 1971
G. Burroughs Mider Lectureship, National Institutes of Health, 1973
Membership, National Academy of Sciences, 1982
American Academy of Arts and Sciences, 1997
Fellow, American Academy of Microbiology, 1997
Fellow, American Association for the Advancement of Science, 1997
International Feltrinelli Prize for Medicine, 2009
Nathan Davis Award of the AMA for Government Service, 2010
Membership, Institute of Medicine of the National Academies, 2010
AACR Team Science Award, 2014
Humbert Humphrey Award, DHHS, 2019
News
Sammies finalist develops new drug for rare form of leukemia
Government Matters 2020.06.18 episode
Dr. Ira Pastan, Distinguished Investigator at the National Cancer Institute at NIH, discusses a drug he developed for a rare type of leukemia and its potential to treat other cancers.
Moxetumomab Approved by FDA for Hairy Cell Leukemia
September 14, 2018, by NCI Staff
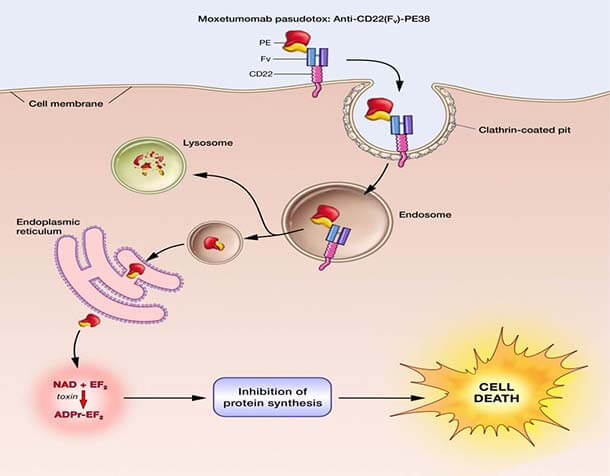
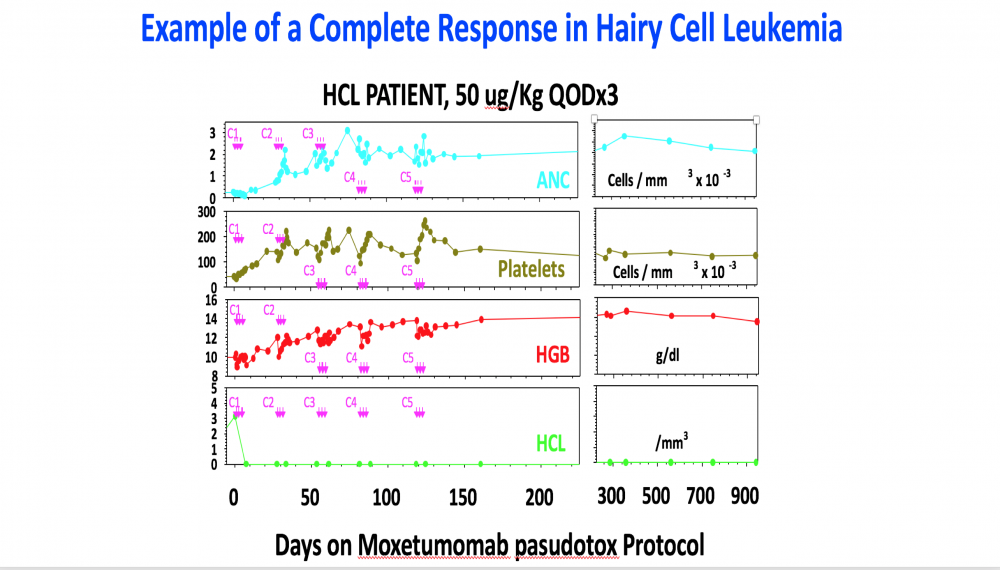
The Food and Drug Administration (FDA) has approved moxetumomab pasudotox (Lumoxiti), a bacterial toxin–based drug, for the treatment of some patients with hairy cell leukemia (HCL). The approval covers the use of moxetumomab in patients with HCL who have already undergone at least two lines of standard treatments.
The action by FDA makes moxetumomab the first treatment approved for this group of patients. The approval was based on the findings from an 80-patient clinical trial sponsored by the drug’s manufacturer, MedImmune.
In the trial, approximately 30% of patients had a complete disappearance of their cancer (complete response) that lasted for a long period, and side effects from the therapy were few and mostly minor. Overall, 75% of patients in the trial had either a partial response or complete response.
Moxetumomab was originally discovered by Ira Pastan, M.D., and colleagues in NCI’s Center for Cancer Research (CCR), and later licensed to MedImmune/AstraZeneca for clinical development.
“This is an important example of conceptually innovative research conducted at NCI that has come to practical fruition with a commercial partner,” said CCR Director Tom Misteli, Ph.D. “It’s the kind of work made possible by our ability at NCI to pursue long-term, high-risk research, including on rare forms of cancers such as hairy cell leukemia. Moxetumomab will make a difference in the lives of patients with this incurable disease.”
Many people diagnosed with HCL will achieve remission with current treatments, explained the trial’s lead investigator, Robert Kreitman, M.D., of CCR's Laboratory of Molecular Biology.
“But about 30%–40% of those patients will relapse 5 to 10 years after their first treatment,” Dr. Kreitman continued.
As patients receive further treatments, although they may go into remission again, the length of those remissions gets shorter and shorter, and the toxicities of the treatments accumulate, he explained. For these patients, few effective treatments exist, which is what makes this new approval so important.
“Moxetumomab represents a promising nonchemotherapeutic treatment for HCL, addressing an unmet medical need for patients,” Dr. Kreitman said.
Moxetumomab includes a special boxed warning for clinicians and patients about the risk of capillary leak syndrome, in which fluid and proteins leak out of tiny blood vessels into surrounding tissues.
Further details on moxetumomab and the clinical trial on which FDA's approval was based are available in this June 2018 Cancer Currents post.
Covers
Oncotarget 24 May 2016 issue cover
Structural models of Recombinant Immunotoxins.
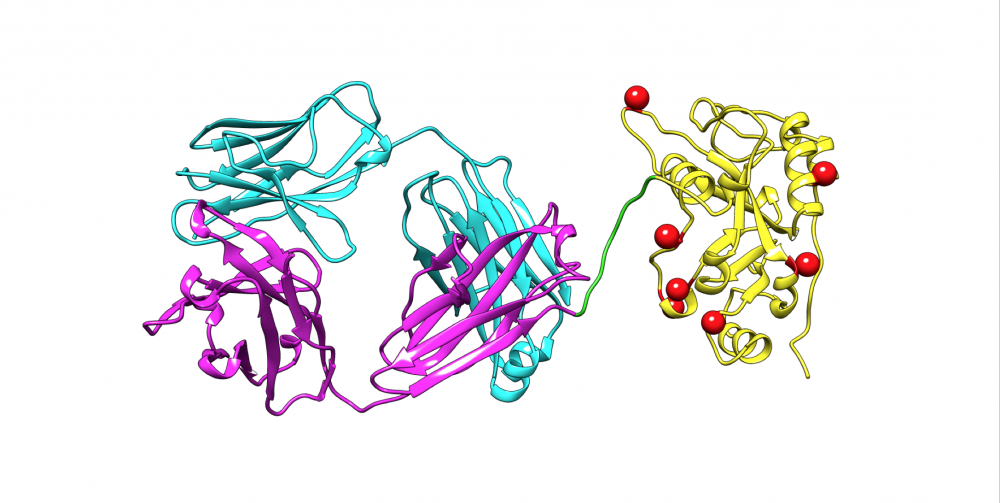
A. SS1P consists of the disulfide-stabilized heavy chain Fv (VH) (magenta) and light chain Fv (VL) (Cyan) of the antibody SS1P. The VH is linked to a 38-kDa fragment of PE38 that is divided into domain II (gray), domain III (yellow), and part of domain Ib from native PE38. B. SS1-LO10R. 24-kDa fragment of PE24 with six point mutations in domain III designed to eliminate binding to B-cell receptor. Point mutations are marked with red balls. C. LMB-T20. PE24 with six point mutations in domain III designed to diminish T-cell epitopes. D. LMB-T14. PE24 with 10 point mutations in domain III designed to diminish B and T cell epitopes. All models are hypothetical arrangements based on the structures of native PE and immunoglobulin G; they do not represent actual structure determinations.
Ronit Mazor1, Masanori Onda1, Dong Park1,3, Selamawit Addissie1, Laiman Xiang1,*, Jingli Zhang2, Raffit Hassan2 and Ira Pastan1
1 Laboratory of Molecular Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA
2 Thoracic and GI Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA
3 New Business Development Department, Medytox Inc., Bundang-gu, Seongnam-si, Gyeonggi-do, South Korea
Oncotarget, Vol. 7, No. 21