NF1
The POB NF team studies NF1 and develops novel clinical trials to find treatments for NF1-related plexiform neurofibromas (PNs) and malignant peripheral nerve sheath tumors (MPNST). We/they team strive to improve clinical trial design to facilitate the study and discovery of treatments for NF.
Natural History of Plexiform Neurofibromas
Treatment trials for NF1 began in the early 2,000s, but scientists didn’t fully understand how fast and when NF1 tumors grow, and if they could shrink on their own without any treatment. To address this, in 2008, NCI scientists launched the Natural History Study of Patients with NF1 (NCT00924196).
Unlike a treatment trial, which looks at the effects of a drug or other intervention, a natural history study focuses on how a disease progresses and how to treat it. The NCI study is still ongoing after more than a decade and led to many advances in our understanding of NF1 and its many manifestations.
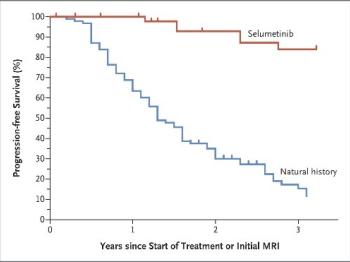
A key innovation to come out of this trial is the use of volumetric, or 3-dimensional, magnetic resonance imaging (MRI) rather than one or two-dimensional measurements to monitor for changes in PN growth. Volumetric MRI allowed researchers to define the natural growth patterns of PN, including the fact that they grow most quickly in young children.
Perhaps most excitingly, the information we have learned from the NF1 Natural History Study was a key part of the application to the FDA that led to the MEK inhibitor selumetinib being the first approved medication for patients with NF1 and PNs. Information from the natural history study that showed the pattern of growth in untreated PNs helped to prove that treatment with selumetinib was truly making a difference for patients.
Treatment Trials for Plexiform Neurofibromas at the POB
Since 2001, Dr. Brigitte Widemann and the POB NF1 team have been designing clinical trials to test drugs for NF, with the hopes of finding an effective treatment for kids with NF1. One of the first trials was for a drug called tipifarnib. This innovative trial was a double-blind, randomized, placebo-controlled study with a cross-over design which meant that all participants with tumor growth had access to the study drug. Though this study did not show that tipifarnib slowed the growth of or shrank the PN, the data obtained from the placebo arm of the trial has been used as a comparison for all subsequent treatment trials(5).
In 2011, Dr. Widemann and the NF team began the SPRINT study (Selumetinib for Plexiform Neurofibromas Trial), a multi-center phase I study of the MEK inhibitor, selumetinib, for children with NF1 and inoperable PNs. The initial results from the phase I study were published in the New England Journal of Medicine in 2016 and showed that selumetinib was the first medication to shrink most of these tumors(6).
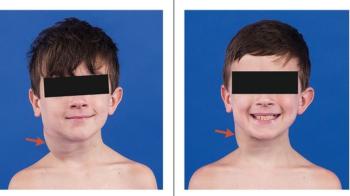
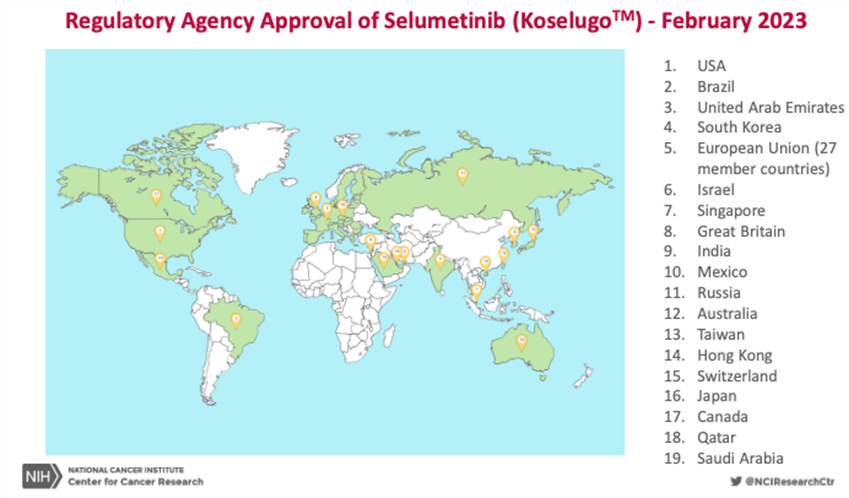
This led to the larger phase II study of selumetinib for symptomatic, inoperable PN. The results, which were also published in the New England Journal of Medicine, show that about 70% of patients saw their tumors shrink and many patients also saw improvements in their pain levels, mobility and physical functioning. In April 2020, the FDA approved selumetinib as the first treatment available for children (ages 2 years and older) with NF1 and inoperable PNs.
This work also highlights the importance of using clinical outcomes other than tumor shrinkage to gain FDA approval for treatments for PNs (and other rare tumors). The NF team used patient reported outcomes (PRO) data to show that the majority of children treated with selumetinib saw improvements in quality-of-life measures, such as pain, mobility and physical functioning.
Read more about the story of selumetinib and the NF team in this NCI blog post.
In addition, Dr. Widemann, along with Dr. Alice Chen and Dr. Geraldine O’Sullivan Coyne, from the NCI’s Developmental Therapeutics Clinic, are testing selumetinib in adults with NF1-related PN. Preliminary results from the ongoing trial show that the treatment shrank tumors in the majority of adults and lessened pain.