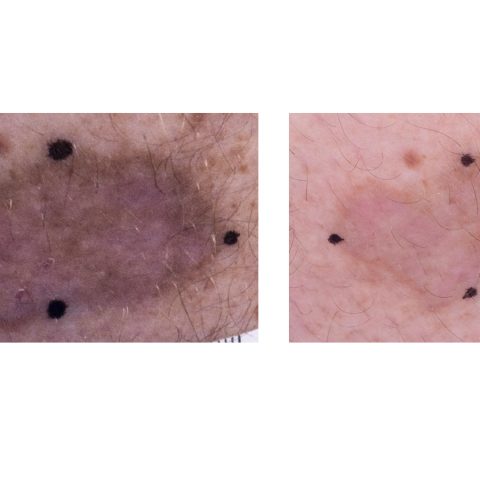
Baseline to 6 months on KS clinical trial treatment on NHS-IL12 study
Adults with Kaposi sarcoma may be eligible to participate in a clinical trial at the NIH Clinical Center.
Kaposi sarcoma (KS) is a cancer that causes patches of abnormal tissue, called lesions, to grow under the skin or in other organs. The lesions are red or purple and usually appear on the legs or face, often causing no symptoms. However, lesions in the lungs, liver, or digestive tract can be life-threatening. KS, caused by a virus known as Kaposi sarcoma herpesvirus (KSHV), is common in people infected with the human immunodeficiency virus (HIV) but can occur in people who do not have HIV. Ramya Ramaswami, M.B.B.S., M.P.H., Physician-Scientist Early Investigator in the HIV and AIDS Malignancy Branch, is leading a study to see if a drug that is currently used to treat patients with breast cancer can help people with KS. Abemaciclib works by blocking the action of an abnormal protein that signals cancer cells to multiply. Research from the Yarchoan Lab has shown that abemaciclib impacts the growth of KSHV-infected cells and can enhance expression of immune surface markers on these cells. Other studies have also shown that this family of drugs impact T-cell immunity to better recognize certain cancers. The goal of this study is to find a safe dose of abemaciclib for people with KS and to see if it can treat and reduce the burden of KS lesions.
Clinicaltrials.gov identifier: NCT04941274
NCI Protocol ID: NCI-21-C-0026
Official Title: A Phase I/II Study of Abemaciclib in Patients With HIV-associated and HIV-negative Kaposi Sarcoma
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.