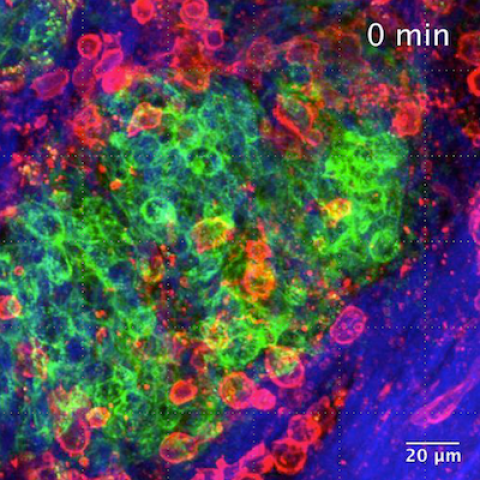
Snapshot from live imaging of harvested gastrointestinal stromal tumor tissue on the SMART system.
Image Credit: Kirsten Remmert, PhD and Jonathan Hernandez, MD
People with biopsy-confirmed gastrointestinal stromal tumors (GISTs), or who have symptoms that suggest GIST, may be eligible to participate in a clinical trial at the NIH Clinical Center.
Andrew M. Blakely, M.D., Assistant Research Physician in the Surgical Oncology Program, is leading a study of GIST that could benefit current and future patients. More than half of GISTs start in the stomach, but they can start anywhere in the GI tract. GISTs start in very early forms of special cells in the wall of the GI tract that signal the muscles of the GI tract to contract to move food and liquid forward. Targeted therapies have improved GIST treatment over the last 20 years. However, some patients’ tumors lack the necessary genetic changes to respond to those drugs, and other patients’ tumors become resistant over time. Therefore, the primary treatment for GISTs is to surgically remove the tumors. Most patients will need numerous surgeries to remove new tumors as they develop. Much more research is needed to understand the genetics of GIST and to identify or develop new drug therapies. Patients enrolled in this study will be evaluated to see if surgery is appropriate for them. If it is, they can have the surgery at the NIH Clinical Center. Otherwise, researchers will reevaluate them every six months until surgery does seem appropriate. These surgeries will remove (or reduce the size of) tumors and will help researchers by providing fresh tumor tissue for further studies.
Clinicaltrials.gov identifier: NCT04557969
NCI Protocol ID: NCI-20-C-0161
Official Title: Prospective Study of Surgery in Gastrointestinal Stromal Tumors (GISTs) for Treatment, Tumor Modeling, and Genomic Analysis
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.


