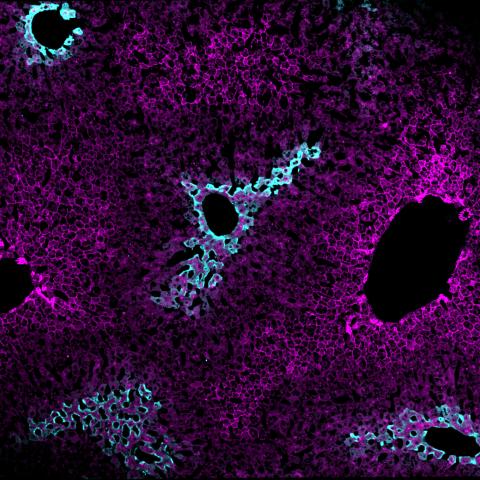
Immunofluorescence staining of a liver section showing the zonal distribution of hepatocytes near veins and arteries that carry blood into the liver (magenta) and hepatocytes near veins that carry blood away from the liver (cyan). Image credit: Lauryn Brown
Close to a hundred years ago, scientists made a peculiar observation that adjacent liver cells, called hepatocytes, organize into clustered zones that differ in their appearance under the microscope. Now, CCR researchers have found that this hepatocyte diversity is shaped in part by the nutrient-sensing ability of their mitochondria. The discovery, published February 28, 2024, in Nature Communications, has important implications for understanding how cells take on different characteristics in the same tissue — a hallmark of many tumors.
“Tumor heterogeneity is a big problem in cancer biology because different cells have different biological behaviors, and this can cause resistance to treatment,” explained Natalie Porat-Shliom, Ph.D., Stadtman Investigator in the Thoracic and GI Malignancies Branch and lead researcher on the study. “Mechanistically, we don’t really understand what gives rise to heterogeneity and how to treat it, but the reality is that normal tissues are also extremely heterogeneous.”
This is a fact, she explained, that is often disregarded when scientists study liver tissue by putting samples in a tube and collecting data from all the cells combined. Porat-Shliom’s team, however, took a different approach to understanding the liver’s complexity. Using a cutting-edge technique called intravital microscopy, they examined the livers of living, anesthetized mice under the microscope.
Homing in on the mitochondria using a mitochondria-targeting fluorescent tag, they found two types of mitochondria in the liver: one type in hepatocytes near arteries and veins that carry blood into the liver for processing, and another type of mitochondria in hepatocytes near veins that carry blood away from the liver.
When they looked more closely at the proteins in the two types of mitochondria — by removing the livers and separating the cells — they found that not only did the mitochondria house different sets of proteins, but even the way the proteins were modified to affect their function differed. The team showed that these differences stemmed from mitochondrial nutrient sensing and could be shifted with drugs that manipulate the mitochondria’s nutrient-sensing activity.
“You can imagine the mitochondria as a communication hub,” Porat-Shliom explained. They are constantly sensing nutrient signals from the environment and translating that into different mitochondrial activities and metabolic output. This, she continued, affects the behavior and appearance of the cells and their mitochondria based on their location in the organ.
Now, the team is collaborating with clinicians to ask if cancers that spread to the liver tend to invade one zone of hepatocytes more so than another. Additionally, they are actively investigating how this liver zonation in the mouse compares to liver cell organization in humans.
Ultimately, Porat-Shliom’s hope is to relate the team’s findings about mitochondrial zonation in the mouse model to what is happening in human disease. “The first step is to understand how things normally work. Then you can compare and contrast that with what goes wrong,” she said.