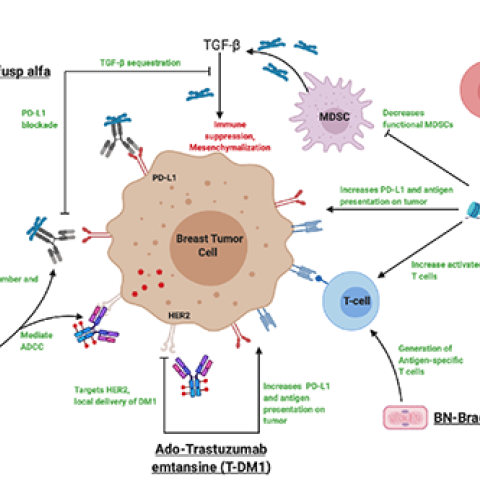
The combination of immunotherapy agents can augment key components of a successful anti-tumor immune response.
Image source: Margaret Gatti-Mays, M.D. (made via Biorender)
Adults who have been diagnosed with metastatic breast cancer may be eligible to participate in a clinical trial at the NIH Clinical Center.
Fatima Karzai, M.D., Associate Research Physician in the Genitourinary Malignancies Branch, is leading a study in adults with breast cancer that has spread to other places in the body. Researchers want to see if a combination of four drugs, which includes immunotherapy, can shrink the tumors of metastatic breast cancer. Participants will be assigned to 1 of 3 groups, with all participants receiving BN-brachyury and M7824. M7824 blocks two signaling pathways that cancer cells use to avoid being killed by the immune system. Researchers are studying the BN-Brachyury cancer vaccine to see if it will help the immune system kill tumor cells and slow the growth of cancer cells. Some participants will get i.v. ado-trastuzumab emtansine, which targets cancer cells that express a protein called HER2 which causes cell death. Finally, some participants will get entinostat in tablet form. Entinostat may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. The goal of the study is to observe how participants respond to the study drugs and the safety of these combinations.
Clinicaltrials.gov identifier: NCT04296942
NCI Protocol ID: NCI-20-C-0056
Official Title: A Phase 1b Trial of Sequential Combinations of BN-Brachyury, Entinostat, Ado-trastuzuamb Emtansine and M7824 in Advanced Stage Breast Cancer (BrEAsT)
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.