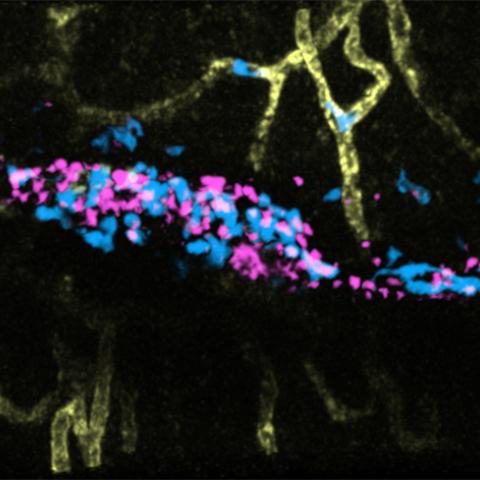
Neutrophils (blue) leaving the vasculature (yellow) to directionally migrate and engage with bacterial bioparticles (magenta) introduced into the hind footpad of a live mouse. Images were acquired using intravital two-photon microscopy.
Image credit: Bhagawat Subramanian
CCR researchers used a state-of-the-art imaging technique to observe the journey neutrophils take when they leave the blood vessels and travel to an area of inflammation, in this case, bacteria introduced into a mouse’s hind footpad. The images revealed that the leukocyte LTB4, and its receptor, BLT1, orchestrate the recruitment and penetration of neutrophils into the area of inflammation, initiating the first immune response to infection. These results published August 26, 2020 in the Journal of Cell Biology.
Roberto Weigert, Ph.D., Senior Investigator, Laboratory of Cellular and Molecular Biology, has been a pioneer in developing the imaging tool, intravital subcellular two-photon microscopy, used in this research. “This is the first time we applied this process to visualize neutrophils at a subcellular resolution in vivo,” says Weigert. “Using multiple colors of fluorescent molecules, we can follow different processes, including how neutrophils leave blood vessels, migrate, and engage with bacteria.”
Although researchers have known for a while that LTB4, and its receptor, BLT1, together known as the LTB4-BLT1 axis, were involved in activating neutrophils, this work uncovered the mechanism through which the axis orchestrates this process. It begins when LTB4 signaling coordinates the distribution of two regulators, non-muscle myosin IIA (NMIIA) and Beta2-integrin (Itgb2). Myosin regulates the mechanical aspect of the process, helping to push the neutrophils through the blood vessels. In this way, neutrophils can penetrate into tissues and reach the inflamed area. The second regulator, Itgb2, helps neutrophils to firmly attach to the cells lining the blood vessel. As inflammation persists, the neutrophils release extracellular vesicles, which adhere to the blood vessels, attracting more neutrophils to the area and building a robust response.
Weigert and his team have also been looking at the role of inflammation and the immune response in cancer. In other ongoing studies involving a head-and-neck mouse model, Weigert’s team tracked how neutrophils responded when the origin of the inflammation was a tumor.
“The more we understand how these mechanisms work in living organisms, the greater the possibility of learning how to modulate inflammation and control disease progression,” says Weigert. “These new visualization tools are bringing us closer to the biological edge, even allowing us to view the process of tumor progression and further elucidate the connections between immune cells and inflammation.”
Neutrophils in action during inflammation in vivo
Posted on Wed, 09/09/2020