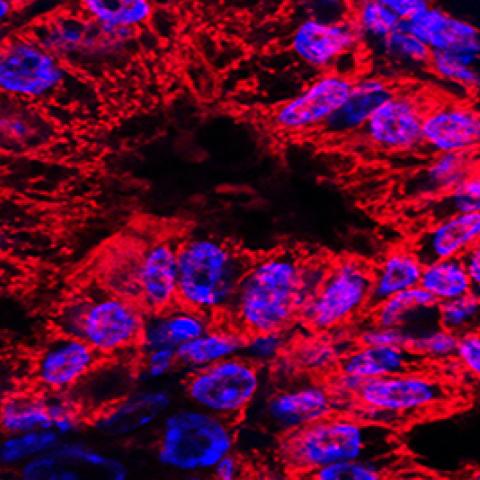
This image shows mitochondrial staining (red) and nuclear staining (blue) of abnormal pancreatic ducts from a mouse model of human pancreatic ductal carcinoma.
Photo courtesy of NCI Visuals Online
A computer analysis has been able to predict that low levels of asparagine, an amino acid required for protein synthesis, combined with the shutdown of a stress response pathway can lead to reductions in the fitness of a tumor—a prediction corroborated through animal and cell line studies. These combined findings could potentially lead to new combination therapies to treat aggressive tumors, such as those found in pancreatic cancers.
Collaborating with the lab of Ze’ev Ronai, Ph.D., Sanford Burnham Prebys Medical Discovery Institute (SBP), CCR researchers ran a computational analysis of data from patients with melanoma and pancreatic cancer and identified a relationship between asparagine and the MAPK (mitogen-activated protein kinases) stress response pathway. The results appeared November 18, 2019, in Nature Cell Biology.
The contribution of the computational analysis, performed by Joo Sang Lee, Ph.D., a member of CCR’s Cancer Data Science Laboratory (CDSL), was to detect synthetic lethal interactions between asparagine and the MAPK stress response pathway and to predict whether this resulted in increased tumor activity. In order to reverse the effect of the synthetic lethal reaction, both variables, the level of asparagine and the activity of the MAPK stress pathway, had to be markedly reduced.
Eytan Ruppin, M.D., Ph.D., Chief of CDSL, noted, “The novelty of the finding was identifying, through synthetic lethality, that one pathway could compensate for a deficiency found in the amino acid asparagine and its concomitant activation could effectively target these tumors.” This information led to comprehensive molecular studies to further test and reveal the mechanisms underlying these predictions.
During the first phase of the lab work, SBP researchers administered a drug called L-asparaginase (approved to treat certain leukemias) to deprive pancreatic tumors in mice of the asparagine needed for protein synthesis. Instead of dying, however, the tumor turned to the MAPK stress pathway to survive. Only after adding a second drug, a MEK inhibitor, did the tumors shrink, proving the initial prediction of a synthetic lethal interaction.
To further confirm these findings, Ruppin and his team conducted additional computational analyses in a subset of patients with two specific cancer types (pancreatic cancer and melanoma) to see if low expression of asparagine and low MAPK activity reduced tumor fitness and led to a more favorable prognosis, as measured by a reduction in tumor size. This proved to be the case, providing more evidence for the potential of clinical applications.
The team is now meeting with clinical researchers to discuss how to advance the use of these two drugs in a preclinical study. “This is welcome news, especially during Pancreatic Cancer Awareness Month,” says Dr. Ruppin.