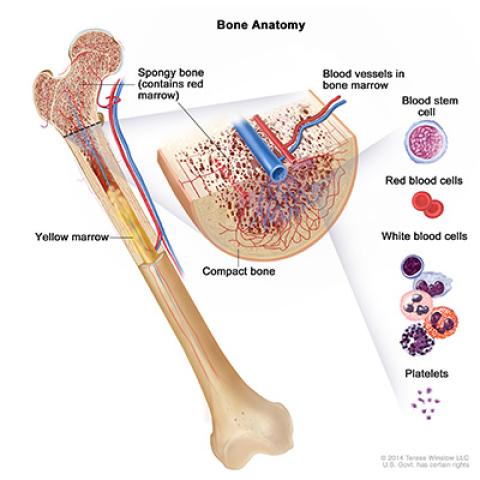
Bone anatomy
Photo courtesy of NCI Visuals Online
People with leukemia, lymphoma or multiple myeloma may be eligible to participate in a new clinical trial at the NIH Clinical Center.
Hematologic malignancies are cancers that begin in the cells of blood-forming tissue, such as bone marrow. Giving a patient bone marrow from a donor (hematopoietic cell transplantation; HCT) is a common treatment for blood cancers. First, doctors must test the patient’s and donor’s blood to find out their human leukocyte antigen (HLA) type. HLAs are proteins found on most cells of the body. Historically, doctors looked for a donor whose HLA type closely matched the patient’s because a fully matched donor previously had a greater chance of success. However, new approaches to transplant, particularly the use of a high dose of the chemotherapy drug cyclophosphamide given early after the transplant, now allows donors who are only half-matched (haploidentical) to be used safely and with outcomes that are similar to using a fully matched donor. This advance now has made HCT available to nearly all patients. The use of cyclophosphamide after HCT (post-transplantation cyclophosphamide) may reduce the risk of severe forms of graft-versus-host disease (GVHD), particularly chronic GVHD. Christopher G. Kanakry, M.D., Investigator in the Experimental Transplantation and Immunology Branch, is conducting a clinical trial to test if an intermediate dose of this drug is better than higher doses as well as evaluating the optimal time to give this drug.
Clinicaltrials.gov identifier: NCT03983850
NCI Protocol ID: NCI-19-C-0112
Official Title: Phase I/II Study De-Intensifying Exposure of Post-Transplantation Cyclophosphamide as GVHD Prophylaxis After HLA-Haploidentical Hematopoietic Cell Transplantation for Hematologic Malignancies
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.