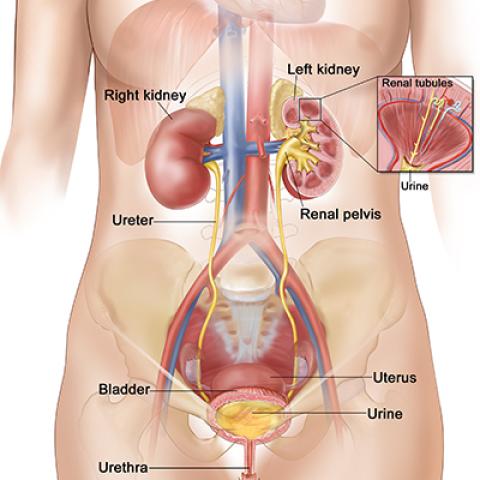
Anatomy of the female urinary system
Photo credit: NCI Visuals Online
Patients with metastatic or advanced urothelial cancer with DNA-repair defects may be eligible to participate in a new clinical trial at the NIH Clinical Center.
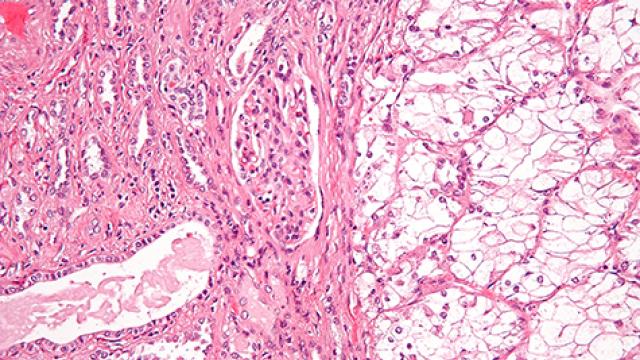
Urothelial carcinoma (UC), or bladder cancer, is cancer of the cells that line the inside of all the organs of the urinary tract (bladder, urethra, ureters and parts of the kidneys). It is the most common cancer of the urinary tract, and it can spread to other parts of the body. Treatment options are limited for patients with metastatic UC. Like every cell in the body, UC cells contain DNA that controls how the cells copy themselves. When this DNA is damaged, the body has ways of repairing it so that the cells can continue to multiply. An enzyme called PARP can repair breaks in DNA and thus keep cells multiplying normally. Andrea B. Apolo, M.D., of the Genitourinary Malignancies Branch, is leading a study of olaparib, a drug designed to target cancers that have defective DNA-repair mechanisms in their cells by inhibiting the action of PARP. Investigators want to test how UC responds to PARP inhibition with olaparib.
Clinicaltrials.gov identifier: NCT03375307
NCI Protocol ID: NCI-19-C-0023
Official Title: A Phase II Study of Olaparib (AZD2281) in Patients With Metastatic/Advanced Urothelial Carcinoma With DNA-Repair Defects
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.