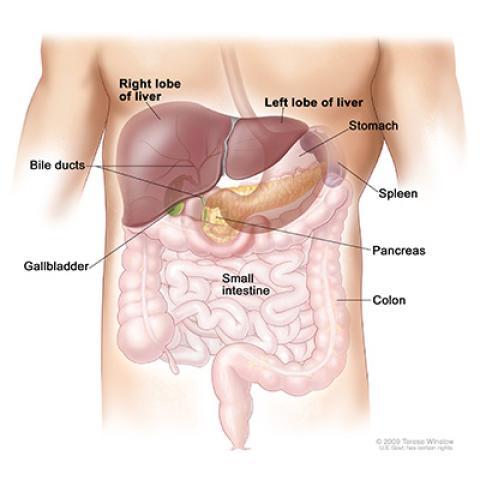
Anatomy of the liver
Photo courtesy of NCI Visuals Online
Patients with fibrolamellar hepatocellular carcinoma, a rare liver cancer, that cannot be treated surgically may be eligible to participate in a new clinical trial at the NIH Clinical Center.
Fibrolamellar hepatocellular carcinoma (FLC) is a rare liver cancer that usually grows in teens and young adults. Under the microscope, FLC appears in the liver as fibrous bands in a plate-like (lamellar) pattern. Unresectable FLC usually does not improve with surgery, chemotherapy or radiation therapy. Tim F. Greten, M.D., Deputy Chief of the Thoracic and GI Malignancies Branch, is leading a study that tests the theory that certain bacteria in the gut may influence the development of FLC. Patients in this study will receive oral vancomycin, an antibiotic. When taken by mouth, vancomycin stays in the intestines where it stops the growth of certain bacteria. In preclinical studies, vancomycin altered gut bacteria, which led to an increase in cancer-fighting cells expressed in the liver. Researchers want to see if vancomycin is safe for humans at a dose higher than that given for a bacterial infection, with no intolerable side effects, and whether it has a beneficial effect on unresectable FLC.
To learn more about this trial, visit our website.
Clinicaltrials.gov identifier: NCT04025567
Official Title: Phase II Study of Oral Vancomycin in Patients With Unresectable Fibrolamellar Hepatocellular Carcinoma (FLC)
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.